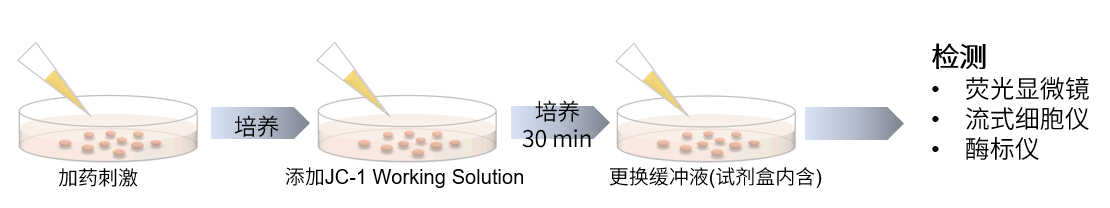
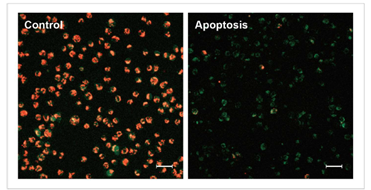
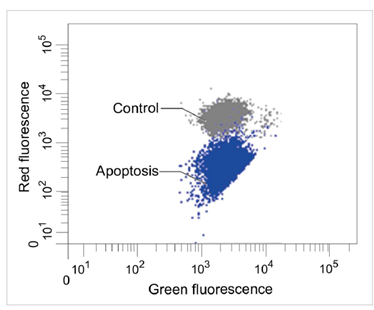
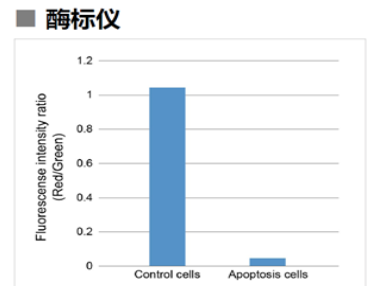
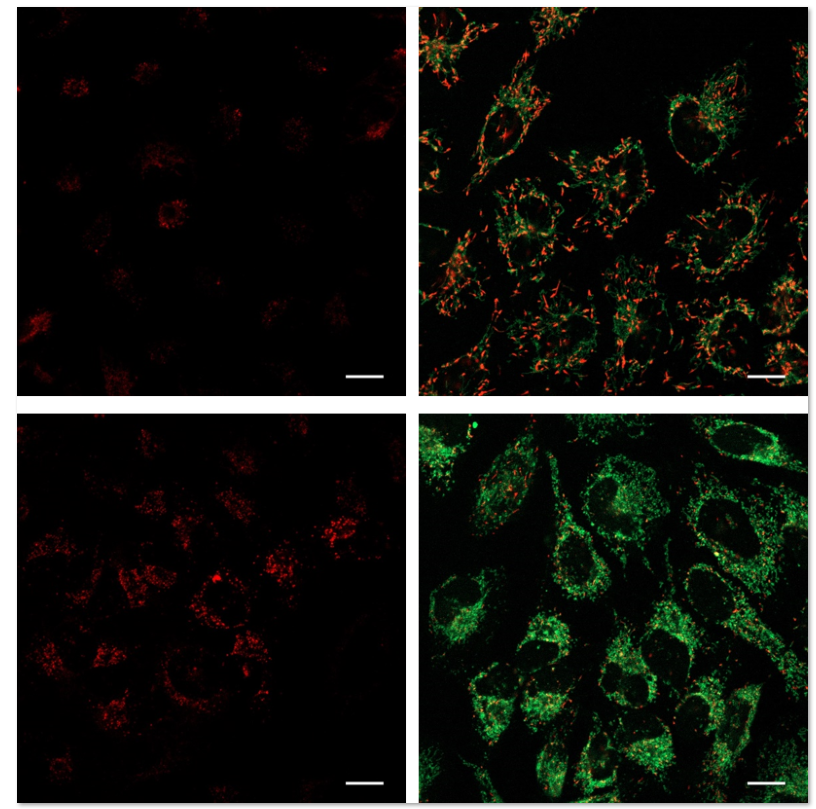
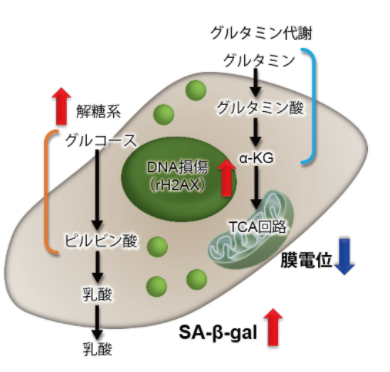
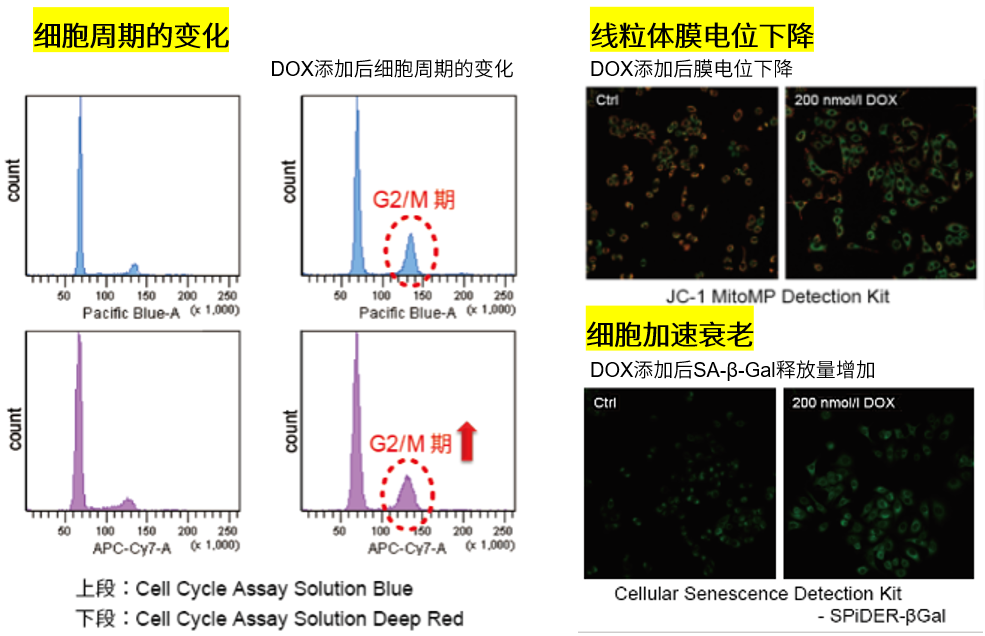
| No. |
Sample Type |
Instrument |
Reference |
| 1 |
Cell:A549 |
Microscope |
K. Li, S. Sun, L. Xiao and Z. Zhang, “Bioactivity-guided fractionation of Helicteres angustifolia L. extract and its molecular evidence for tumor suppression”, Front Cell Dev Biol.,2023, doi: 10.3389/fcell.2023.1157172. |
| 2 |
Cell:A549 |
Flow Cytometer |
C. N. D’Alessandro-Gabazza, T. Yasuma, T. Kobayashi, M. Toda1, A. M. Abdel-Hamid, H. Fujimoto, O. Hataji, H. Nakahara, A. Takeshita, K. Nishihama, T. Okano, H. Saiki, Y. Okano, A. Tomaru, V. F. D’Alessandro, M. Shiraishi, A. Mizoguchi, R. Ono, J. Ohtsuka, M. Fukumura, T. Nosaka, X. Mi, D. Shukla, K. Kataoka, Y. Kondoh, M. Hirose, T. Arai, Y. Inoue, Y. Yano, R. I. Mackie, I. Cann and E. C. Gabazza, “Inhibition of lung microbiota-derived proapoptotic peptides ameliorates acute exacerbation of pulmonary fibrosis”, Nat. Comm., 2022, doi:10.1038/s41467-022-29064-3. |
| 3 |
Cell:A549, HeLa |
Plate reader |
J. Yang, L. Liu, Y. Oda, K. Wada, M. Ago, S. Matsuda, M. Hattori, T. Goto, Y. Kawashima, Y. Matsuzaki and T. Taketani,”Highly-purified rapidly expanding clones, RECs, are superior for functional-mitochondrial transfer”, Stem Cell Res Ther., 2023, doi: 10.1186/s13287-023-03274-y. |
| 4 |
Cell:ALM |
Plate reader |
T. Nechiporuk, S.E. Kurtz, O. Nikolova, T. Liu, C.L. Jones, A. D. Alessandro, R. C. Hill, A. Almeida, S. K. Joshi, M. Rosenberg, C. E. Tognon, A. V. Danilov, B. J. Druker, B. H. Chang, S. K McWeeney and J. W. Tyner , “The TP53 Apoptotic Network Is a Primary Mediator of Resistance to BCL2 Inhibition in AML Cells.”, Cancer Discov, 2019, 9, |
| 5 |
Cell:ARPE-19 |
Flow Cytometer/ |
J. Hamuro, T. Yamashita, Y. Otsuki, N. Hiramoto, M. Adachi, T. Miyatani, H. Tanaka, M. Ueno, S. Kinoshita and C. Sotozono,”Spatiotemporal Coordination of RPE Cell Quality by Extracellular Vesicle miR-494-3p Via Competitive Interplays With SIRT3 or PTEN”, Invest Ophthalmol Vis Sci., 2023, doi: 10.1167/iovs.64.5.9. |
| 6 |
Cell:ARPE-19 |
Microscope |
J. H. Quan, F. F. Gao, H. A. Ismail, J. M. Yuk, G. H. Cha, J. Q. Chu and Y. H. Lee, “Silver Nanoparticle-Induced Apoptosis in ARPE-19 Cells Is Inhibited by Toxoplasma gondii Pre-Infection Through Suppression of NOX4-Dependent ROS Generation”, Int J Nanomedicine., 2020, 15, 3695–3716. |
| 7 |
Cell:C2C12, myocytes |
– |
Z. Jing, T. Iba, H. Naito, P. Xu, J.I. Morishige, N. Nagata, H. Okubo and H.Ando ,”L-carnitine prevents lenvatinib-induced muscle toxicity without impairment of the anti-angiogenic efficacy”, Front Pharmacol., 2023, doi: 10.3389/fphar.2023.1182788. |
| 8 |
Cell:C2C12, 3T3L1 |
Plate reader |
M. Kurano, K. Tsukamoto, T. Shimizu, H. Kassai, K. Nakao, A. Aiba, M. Hara and Yatomi , “Protection Against Insulin Resistance by Apolipoprotein M/Sphingosine 1-Phosphate “, Diabetes, 2020, DOI: 10.2337/db19-0811. |
| 9 |
Cell:Colon 26 |
Microscope |
B. Uranbileg, M. Kurano, K. Kano, E. Sakai, J. Arita, K. Hasegawa, T. Nishikawa, S. Ishihara, H. Yamashita, Y. Seto, H. Ikeda, J. Aoki and Y. Yatomi,”Sphingosine 1‐phosphate lyase facilitates cancer progression through converting sphingolipids to glycerophospholipids”, Clin Transl Med., 2022, doi: 10.1002/ctm2.1056. |
| 10 |
Tissue:
Frozen heart slides |
Microscope |
W. Yu, Y. Hu, Z. Liu, K. Guo, D. Ma, M. Peng, Y. Wang, J. Zhang, X. Zhang, P. Wang, J. Zhang, P. Liu and J. Lu,”Sorting nexin 3 exacerbates doxorubicin-induced cardiomyopathy via regulation of TFRC-dependent ferroptosis”, Acta Pharmaceutica Sinica B., 2023, doi: https://doi.org/10.1016/j.apsb.2023.08.016. |
| 11 |
Cell:HCE |
Microscope |
T. Yamashita, K. Asada, M. Ueno, N. Hiramoto, T. Fujita, M. Toda, C. Sotozono, S. Kinoshita and J. Hamuro,”Cellular interplay through extracellular vesicle miR-184 alleviates corneal endothelium degeneration”, Ophthalmol Sci., 2022, doi: 10.1016/j.xops.2022.100212. |
| 12 |
Cell:HCE |
Microscope |
M. Ueno, K Yoshii, T. Yamashita, K. Sonomura, K. Asada, E. Ito, T. Fujita, C. Sotozono, S. Kinoshita and J. Hamuro,”The Interplay Between Metabolites and MicroRNAs in Aqueous Humor to Coordinate Corneal Endothelium Integrity”, Ophthalmol Sci., 2023, doi: 10.1016/j.xops.2023.100299. |
| 13 |
Cell:HCE-T |
– |
W. Otsu, T. Yako, E. Sugisawa, S. Nakamura, H. Tsusaki, N. Umigai, M. Shimazawa and H. Hara,”Crocetin protects against mitochondrial damage induced by UV-A irradiation in corneal epithelial cell line HCE-T cells”, J Pharmacol Sci., 2022, doi: 10.1016/j.jphs.2022.10.005. |
| 14 |
Cell:HCE-T |
Microscope |
K. Ishida, T. Yako, M. Tanaka, W. Otsu, S. Nakamura, M. Shimazawa, H. Tsusaki and H. Hara,”Free-radical scavenger NSP-116 protects the corneal epithelium against UV-A and blue led light exposure”, Biol Pharm Bull., 2021, doi: 10.1248/bpb.b21-00017. |
| 15 |
Cell:HepG |
Microscope/Spectrophotometer |
M. Ikura, K. Furuya, T. Matsuda and T. Ikura,”Impact of Nuclear De Novo NAD+ Synthesis via Histone Dynamics on DNA Repair during Cellular Senescence To Prevent Tumorigenesis”, Mol Cell Biol., 2022, doi: 10.1128/mcb.00379-22. |
| 16 |
Cell:hiPSCs, Neurons |
Microscope |
T. Hara, M. Toyoshima, Y. Hisano, S. Balan, Y. Iwayama, H. Aono,Y. Futamura, H. Osada, Y. Owada and T. Yoshikawa,”Glyoxalase I disruption and external carbonyl stress impair mitochondrial function in human induced pluripotent stem cells and derived neurons”, Translational Psychiatry., 2021, doi: 10.1038/s41398-021-01392-w. |
| 17 |
Cell:HSCs |
Microscope |
Y. Su, S. Lu, C. Hou, K. Ren, M. Wang, X. Liu, S. Zhao and X. Liu ,”Mitigation of liver fibrosis via hepatic stellate cells mitochondrial apoptosis induced by metformin”, International Immunopharmacology., 2022, doi: 10.1016/j.intimp.2022.108683. |
| 18 |
Cell:HUVECs |
Microscope |
D. Ueno, K. Ikeda, E. Yamazaki, A. Katayama, R. Urata and S. Matoba ,”Spermidine improves angiogenic capacity of senescent endothelial cells, and enhances ischemia-induced neovascularization in aged mice”, Sci Rep., 2023, doi: 10.1038/s41598-023-35447-3. |
| 19 |
Cell:KYSE30 |
Microscope |
Q. Luo, X. Wu, P. Zhao, Y. Nan, W. Chang, X. Zhu, D. Su and Z. Liu,”OTUD1 activates caspase‐independent and caspase‐dependent apoptosis by promoting AIF nuclear translocation and MCL1 degradation”, Adv Sci (Weinh)., 2021, doi: 10.1002/advs.202002874. |
| 20 |
Cell: Macrophage |
Microscope |
G. Yang, M. Fan, J. Zhu, C. Ling, L. Wu, X. Zhang, M. Zhang, J. Li, Q. Yao, Z. Gu and X. Cai, “A multifunctional anti-inflammatory drug that can specifically target activated macrophages massively deplete intracellular H2O2 and produce large amounts CO for a highly efficient treatment of osreoarthritis” , Biomaterials, 2020, doi:10.1016/j.biomaterials.2020.120155. |
| 21 |
Cell:MDA-MB-415, MCF-7 |
Microscope |
S.Y. Park, K.J. Jeong, A. Poire, D. Zhang, Y.H. Tsang, A.S. Blucher and G.B. Mills ,”Irreversible HER2 inhibitors overcome resistance to the RSL3 ferroptosis inducer in non-HER2 amplified luminal breast cancer”, Cell Death & Disease., 2023, doi: 10.1038/s41419-023-06042-1. |
| 22 |
Cell:MIN6 |
Plate reader/Microscope |
N. Mizusawa, N. Harada, T. Iwata, I. Ohigashi, M. Itakura and K. Yoshimoto,”Identification of protease serine S1 family member 53 as a mitochondrial protein in murine islet beta cells”, Islets., 2022, doi: 10.1080/19382014.2021.1982325. |
| 23 |
Cell:MSCs |
Flow Cytometer |
S.Y. Jo, H.J. Cho and T.M. Kim,”Fenoldopam mesylate enhances the survival of mesenchymal stem cells under oxidative stress and increases the therapeutic function in acute kidney injury”, Cell Transplant., 2023, doi: 10.1177/09636897221147920. |
| 24 |
Cell:Neuro-2A |
Microscope、Plate reader |
Y. Wang, Y. Shinoda, A. Cheng, I. Kawahata and K. Fukunaga,”Epidermal fatty acid-binding protein 5 (FABP5) Involvement in alpha-synuclein-induced mitochondrial injury under oxidative stress”, Biomedicines., 2021, doi: 10.3390/biomedicines9020110. |
| 25 |
Cell:Neuron |
Microscope |
I. Kawahata, L. Luc Bousset, R. Melki and K. Fukunaga , “Fatty Acid-Binding Protein 3 is Critical for α-Synuclein Uptake and MPP+-Induced Mitochondrial Dysfunction in Cultured Dopaminergic Neurons “, Int J Mol Sci., 2019, 20, 5358. |
| 26 |
Cell:Neuron |
Microscope |
A. Fukuda, S. Nakashima,Y. Oda, K. Nishimura, H. Kawashima, H. Kimura, T. Ohgita, E. Kawashita, K. Ishihara, A. Hanaki, M. Okazaki, E. Matsuda, Y. Tanaka, S. Nakamura, T. Matsumoto, S. Akiba, H. Saito, H. Matsuda and K. Takata,”Plantainoside B in Bacopa monniera Binds to Aβ Aggregates Attenuating Neuronal Damage and Memory Deficits Induced by Aβ”, Biol Pharm Bull., 2023, doi: 10.1248/bpb.b22-00797. |
| 27 |
Cell:PAECs |
Plate reader |
T. Sakai, H. Takagaki, N. Yamagiwa, M. Ui, S. Hatta and J. Imai,”Effects of the cytoplasm and mitochondrial specific hydroxyl radical scavengers TA293 and mitoTA293 in bleomycin-induced pulmonary fibrosis model mice”, Antioxidants (Basel)., 2021, doi: 10.3390/antiox10091398. |
| 28 |
Cell:PANC-1 |
Plate reader |
W.A. Naime, A. Kimishima, A. Setiawan, J.R. Fahim, M.A. Fouad, M.S. Kamel and M. Arai,”Mitochondrial Targeting in an Anti-Austerity Approach Involving Bioactive Metabolites Isolated from the Marine-Derived Fungus Aspergillus sp.”, Marine drugs., 2020, doi: 10.3390/md18110555. |
| 29 |
Cell:PANC-1, MIAPaca-2 |
Microscope |
T. Taniai, Y. Shirai,Y. Shimada, R. Hamura, M. Yanagaki, N. Takada, T. Horiuchi, K. Haruki, K. Furukawa, T. Uwagawa, K. Tsuboi, Y. Okamoto, S. Shimada, S. Tanaka, T. Ohashi and T. Ikegami,”Inhibition of acid ceramidase elicits mitochondrial dysfunction and oxidative stress in pancreatic cancer cells”, Cancer Sci., 2021, doi: 10.1111/cas.15123. |
| 30 |
Cell:PC |
Flow Cytometer |
R. Hamura, Y. Shirai,Y. Shimada, N. Saito, T. Taniai, T. Horiuchi, N. Takada, Y. Kanegae, T. Ikegami, T. Ohashi and K. Yanaga ,”Suppression of lysosomal acid alpha‐glucosidase impacts the modulation of transcription factor EB translocation in pancreatic cancer”, Cancer Sci., 2021, doi: 10.1111/cas.14921. |
| 31 |
Cell:Porcine oocytes |
Microscope |
W. Hu, Y. Zhang, D. Wang, T. Yang, J. Qi, Y. Zhang, H. Jiang, J Zhang, B. Sun and S. Liang,”Iron Overload-Induced Ferroptosis Impairs Porcine Oocyte Maturation and Subsequent Embryonic Developmental Competence in vitro”, Front Cell Dev Biol., 2021, doi: 10.3389/fcell.2021.673291. |
| 32 |
Cell:Porcine oocytes |
Microscope |
Y. Xiao, B. Yuan, W. Hu, J. Qi, H. Jiang, B. Sun, J. Zhang and S. Liang,”Tributyltin Oxide Exposure During in vitro Maturation Disrupts Oocyte Maturation and Subsequent Embryonic Developmental Competence in Pigs”, Front Cell Dev Biol., 2021, doi: 10.3389/fcell.2021.683448. |
| 33 |
Cell:RGC-5 |
Plate reader |
Y. Aoyama, S. Inagaki, K. Aoshima, Y. Iwata, S. Nakamura, H. Hara and M. Shimazawa,”Involvement of endoplasmic reticulum stress in rotenone-induced leber hereditary optic neuropathy model and the discovery of new therapeutic agents”, J Pharmacol Sci . .,2021, doi: 10.1016/j.jphs.2021.07.003. |
| 34 |
Cell:SAS,HSC-2 |
Plate reader |
K. Yamana, J. Inoue, R. Yoshida, J. Sakata, H. Nakashima, H. Arita, S. Kawaguchi, S. Gohara, Y. Nagao, H. Takeshita, M. Maeshiro, R. Liu, Y. Matsuoka, M. Hirayama, K. Kawahara, M. Nagata, A. Hirosue, R. Toya, R. Murakami, Y. Kuwahara, M. Fukumoto and H. Nakayama,”Extracellular vesicles derived from radioresistant oral squamous cell carcinoma cells contribute to the acquisition of radioresistance via the miR‐503‐3p‐BAK axis”, J Extracell Vesicles., 2021, doi: 10.1002/jev2.12169. |
| 35 |
Cell:SBC-3 |
Flow Cytometer |
N. Takahashi, T. Iguchi, M. Kuroda, M. Mishima and Y. Mimaki,”Novel Oleanane-Type Triterpene Glycosides from the Saponaria officinalis L. Seeds and Apoptosis-Inducing Activity via Mitochondria”, Int J Mol Sci., 2022, doi: 10.3390/ijms23042047. |
| 36 |
Cell:SH-SY5Y |
Microscope |
Q. Guo, I. Kawahata, A. Cheng, H. Wang, W. Jia, H. Yoshino and K. Fukunaga,”Fatty acid-binding proteins 3 and 5 are involved in the initiation of mitochondrial damage in ischemic neurons”, Redox Biology., 2023, doi: 10.1016/j.redox.2022.102547. |
| 37 |
Cell:SiHa |
Microscope |
F.F. Gao, J.H. Quan, M.A. Lee, W. Ye, J.M. Yuk, G.H. Cha, I.W. Choi and Y.H. Lee,”Trichomonas vaginalis induces apoptosis via ROS and ER stress response through ER–mitochondria crosstalk in SiHa cells”, Parasites &vectors., 2021, doi: 10.1186/s13071-021-05098-2. |
| 38 |
Cell:SU-DHL-2 |
Flow Cytometer |
Q. Zhao, D. Jiang, X. Sun, Q. Mo, S. Chen, W. Chen, R. Gui and X. Ma,”Biomimetic nanotherapy: core–shell structured nanocomplexes based on the neutrophil membrane for targeted therapy of lymphoma”, J Nanobiotechnology., 2021, doi: 10.1186/s12951-021-00922-4. |
| 39 |
Cell:THP-1 |
Microscope |
W. Zheng, Z. Zhou, Y. Rui, R. Ye, F. Xia, F. Guo, X. Liu, J. Su, M. Lou, and X.F. Yu,”TRAF3 activates STING-mediated suppression of EV-A71 and target of viral evasion”, Signal Transduct Target Ther., 2023, doi: 10.1038/s41392-022-01287-2. |
| 40 |
Cell:TSM15 |
In Cell Analyzer |
M. Honda, F. Shimizu, R. Sato, Y. Mizukami, K. Watanabe, Y. Takeshita, T. Maeda, M. Koga and T. Kanda,”Jo-1 Antibodies From Myositis Induce Complement-Dependent Cytotoxicity and TREM-1 Upregulation in Muscle Endothelial Cells”, Neurol Neuroimmunol Neuroinflamm., 2023, doi: 10.1212/NXI.0000000000200116. |
| 41 |
Cell:tumor |
Flow Cytometer |
H. Wang, X. Rong, G. Zhao, Y. Zhou, Y. Xiao, D. Ma, X. Jin, Y. Wu, Y. Yan, H. Yang, Y. Zhou, M. Qian, C. Niu, X. Hu, D.Q. Li, Q. Liu, Y. Wen, Y.Z. Jiang, C. Zhao and Z.M. Shao ,”The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer”, Cell Metab., 2022, doi: 10.1016/j.cmet.2022.02.010. |
| 42 |
Cell:TY10 |
In Cell Analyzer |
F. Shimizu, R. Ogawa, Y. Mizukami, K. Watanabe, K. Hara, C. Kadono, T. Takahashi, T. Misu, Y. Takeshita, Y. Sano, M. Fujisawa, T. Maeda, I. Nakashima, K. Fujihara and T. Kanda,”GRP78 antibodies are associated with blood-brain barrier breakdown in anti–myelin oligodendrocyte glycoprotein antibody–associated disorder”, Neurol Neuroimmunol Neuroinflamm., 2022, doi: 10.1212/NXI.0000000000001038. |
| 43 |
Cell:U2OS, HeLa |
Microscope |
T. Namba, “BAP31 regulates mitochondrial function via interaction with Tom40 within ER-mitochondria contact sites “, Sci Adv., 2019, 5, (6), 1386. |