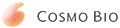Protocol
A) Thawing of Cells
1) Prepare a 100mm dish (Note: 100mm dish is recommended).
2) Warm culture medium to 37°C.
3) Prepare a conical tube (for 15mL) added 10mL of culture medium.
4) Carefully remove the cryovial from liquid nitrogen and thaw cells in a water bath at 37°C for 90 seconds.
5) Transfer the cryovial into a laminar flow hood. Before opening, wipe the outside of the vial with 70% ethanol.
6) Gently transfer the thawed cell suspension (1mL) into 10 mL of culture medium.
7) Transfer 1mL of culture medium in the same conical tube back to the cryovial and pour the contents back to 15mL conical
tube.
8) Centrifuge the cell suspension at approximately 200 ×g for 5 minutes at 4°C.
9) Aspirate the supernatant without disrupting the pellet and resuspend the cells in 10mL of culture medium.
10) Transfer the cell suspension to 100mm dish and incubate the cells in 37°C, 5% CO2 incubator.
11) Replace the medium with fresh pre-warmed culture medium every 2 to 3 days.
B) Subculturing Note: Allow culture medium, HBSS(or PBS(-)), and 0.25% Trypsin to come to room temperature before use.
1) When the cells reach 70 -90% of confluent, they should be subcultured.
2) Aspirate the medium. Rinse the dish with 10mL of HBSS or PBS (-).
3) Add 1mL of 0.25% Trypsin, then incubate at 37°C for 4-6 minutes.
4) Add 10mL of culture medium and disperse the cells with gentle pipetting.
5) Transfer the cell suspension to conical tube and centrifuge at 200 ×g for 5 minutes at 4°C.
6) Aspirate the supernatant without disrupting the pellet and resuspend the cells in 10mL of culture medium.
7) Dilute the cell suspension by adding culture medium. A subcultivation ratio of 1:6 to 1:8 is recommended.
8) Transfer the cell suspension to new 100mm dish and Incubate the cells in 37°C, 5% CO2 incubator.
9) Replace the medium with fresh pre-warmed culture medium every 2 to 3 days.
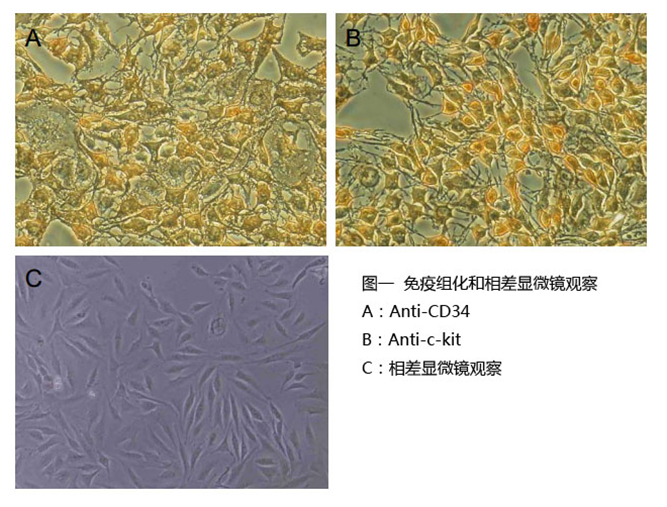
10) Culture the cells until the required density (70 -90% of confluent; Fig 1, C) is reached.
 CSR_GIST-T1CultureKit.pdf
CSR_GIST-T1CultureKit.pdf