上海金畔生物科技有限公司代理日本同仁化学 DOJINDO代理商全线产品,欢迎访问官网了解更多信息
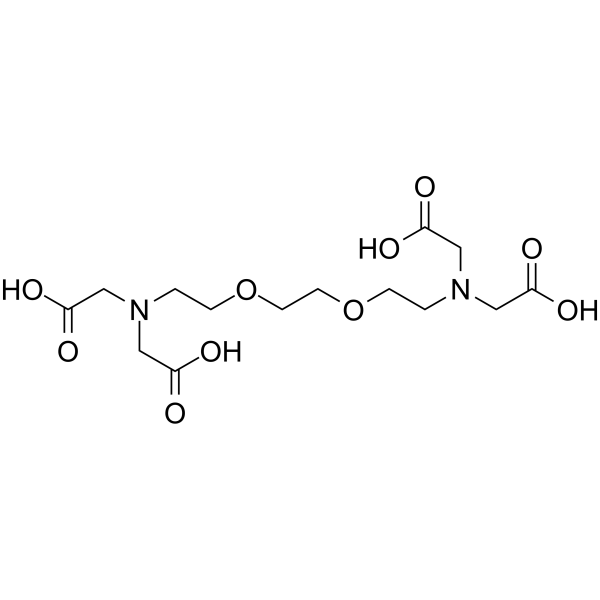
C14H24N2O10
380.35
特点:
● 应用广泛
● 与钙离子结合后稳定
性质
乙二醇醚二胺四乙酸(也缩写为EGTA)。 它比EDTA在有机溶剂(DMF)中的溶解度更高。 它的螯合形成常数与EDTA的螯合形成常数没有太大区别,但是由于它的特异性取决于金属,因此可以选择性地滴定Cd / Zn混合样品中Cd的选择性滴定或Ca和Mg共存的Ca的选择性滴定作为滴定度。试剂,可以。 此外,它还广泛用作电位滴定的生化试剂,此外还用作滤纸色谱中的显影液,酶活性,生物膜和肌肉生理机制的研究。
参考文献
1) C. L. Luke and M. E. Campbell, “Photometric Determination of Magnesium in Electronic Nickel”, Anal. Chem. , 1954, 26, 1778. 2) F. S. Sadek, R. W. Schmid and C. N. Reilley, “Visual EGTA Titration of Calcium in Thepresence of Magnesium”, Talanta, 1959, 2, 38.
3) R. A. Burg and H. F. Conaghan, “Chelometric Determination of Calcium and Magnesium in Minerals”, Chem. Anal. , 1960, 49, 100.
4) C. D. Dwivedi, K. N. Munshi and A. K. Dey, “Photometric Determination of Gallium, Indium, and Thallium Employing 4-(2-Pyridylazo)resorcinol”, Chem. Anal., 1966, 55, 13.
5) F. W. Czech and M. J. McCarthy, “Determination of Acetyl Peroxide in Aqueous Systems Containig Peracetic Acid and Hydrogen Peroxide”, Chem. Anal., 1966, 55, 11.
6) R. Pribil and V. Vesely, “Contributions to the Basic Problems of Complexometry-XX Determination of Calcium and Magnesium”, Talanta, 1966, 13, 233.
7) 伊藤敦子, 上野景平, “ヒドロキシナフトールブルー(HNB)指示薬を用いるカルシウム、マグネシウムの連続キレート滴定”, Jpn. Anal., 1970, 19, 393.
8) 丸山工作, “筋収縮の研究とキレート剤の役割”, Dojin News, 1981, 18, 1.
9) T. Tatsumi and H. Fliss, “Hypochlorous Acid Mobilizes Intracellular Zinc in Isolated Rat Heart Myocytes”, J. Mol. Cell. Cardiol., 1994, 26(4), 471.
10) H. Ohata, Y. Ujiki and K. Momose, “Confocal Imaging Analysis of ATP-Induced Ca2+ Response in Individual Endothelial Cells of the Artery in Situ“, Am. J. Physiol.,1997, 272(6), 1980.
11) I. Sakabe, S. Paul, W. Dansithong and T. Shinozawa, “Induction of Apoptosis in Neuro-2A Cells by Zn2+ Chelating”, Cell Struct. Funct., 1998, 23(2), 95.
常见问题Q&A
| 如何进行溶解? |
| 由于该产品为游离酸形式,因此几乎不溶于水。可使用碱性水溶液(例如0.1N NaOH)溶解后,再配置为试剂水溶液并使用。
GEDTA会被碱中和并溶解。 1mol 的GEDTA会完全溶解于2mol 的碱中,此时的pH值约为5到6。 |
规格性状
性状:
本品为白色结晶性粉末,溶于碱。
纯度(滴定度):97.0%以上
碱溶性:测试合格
灼烧残渣(硫酸盐):0.10%以下
重金属(以铅计):0.001%以下
铁(Fe):0.001%以下