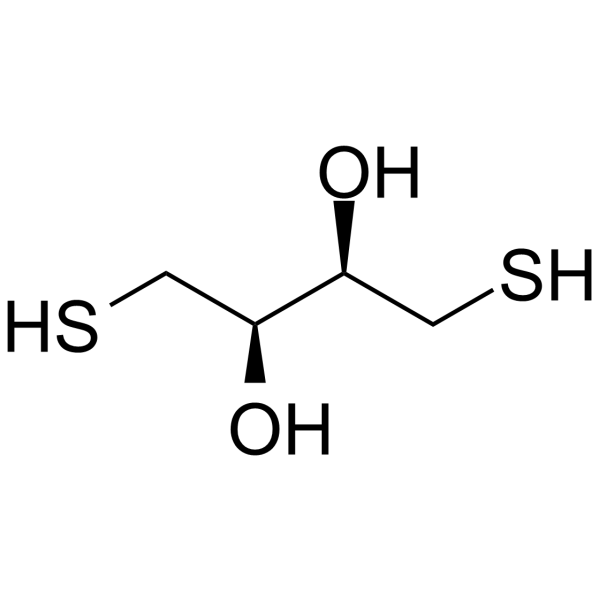
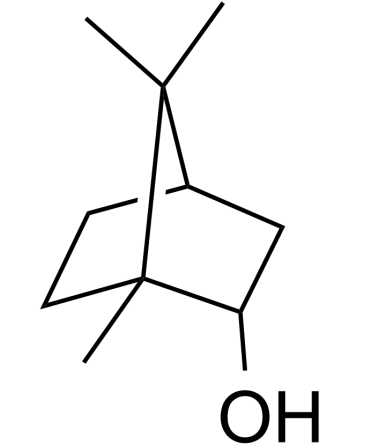
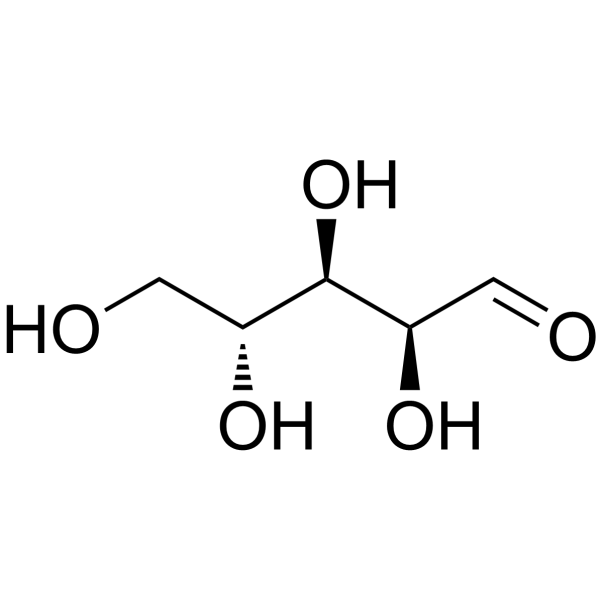
Rats: The pharmacokinetic study is performed 24 h after reperfusion in the model groups, i.e., 26 h after operation in the SO group. The XNJ subgroup rats are orally administered with XNJ decoction dissolved in 0.7% CMC-Na aqueous solution (10.00 ml/kg body weight (BW)). The pure DL-Borneol subgroup also receives gavages of DL-Borneol suspension (10.00 ml/kg BW). DL-Borneol and XNJ suspensions are administrated respectively at a dosage of 162.0 mg/kg of DL-Borneol. Then 0.5 ml plasma samples are collected into heparinized tubes by the puncture of the retro-orbital sinus at 5, 10, 20, 30, 45, 60, 90, 120, 180, 240, and 360 min separately following oral administration. After centrifugation at 6000 r/min for 10 min, plasma samples are stored at −20 °C and analyzed within one week[3].
Mice: Repeated administration of a subconvulsive dose of PTZ (35 mg/kg, i.p.) on every alternate day for 4 weeks produces kindling in mice. DL-Borneol (5, 10, and 25 mg/kg, i.p.) and diazepam (1 mg/kg, i.p.) are given as a pretreatment prior to each PTZ injection during the progression of kindling. Oxidative stress parameters such as superoxide dismutase (SOD), reduced glutathione (GSH), catalase (CAT), and lipid peroxidation (LPO) are assessed at the end of the study. Neuronal damage is assessed by hematoxylin and eosin staining technique. GFAP is also evaluated in the hippocampus region of the brain by using immunohistochemistry[2].
Shanghai Jinpan Biotech Co Ltd has not independently confirmed the accuracy of these methods. They are for reference only.





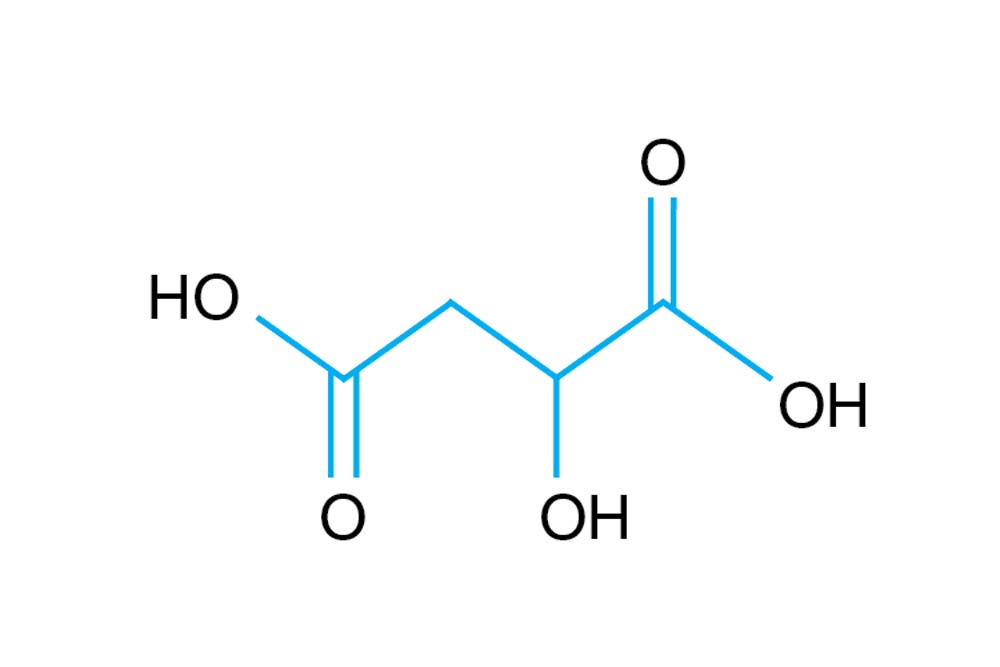
 HR2-761 DL-Malic acid pH 7.0 SDS
HR2-761 DL-Malic acid pH 7.0 SDS