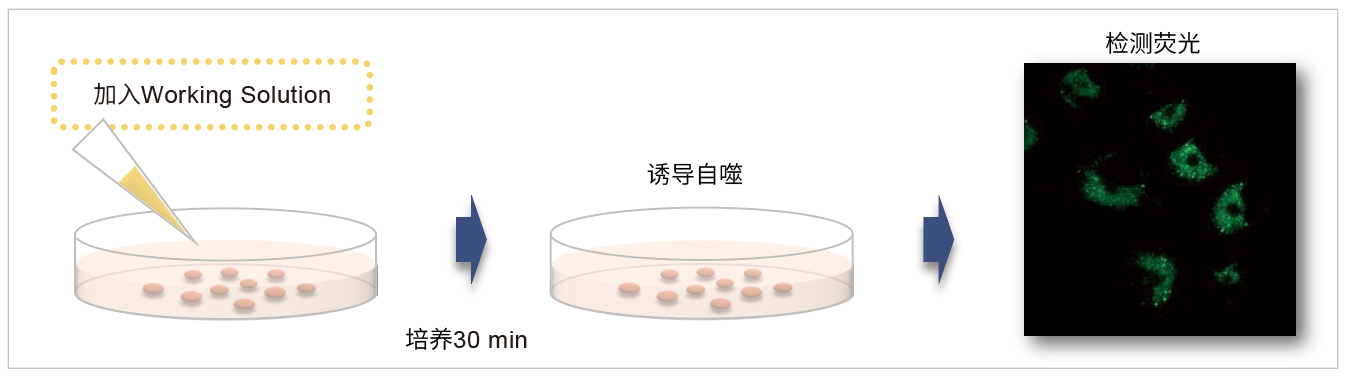
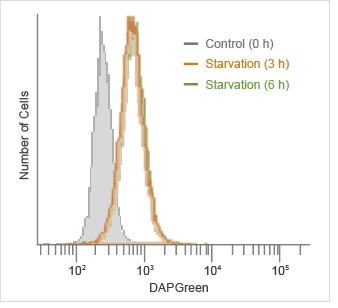
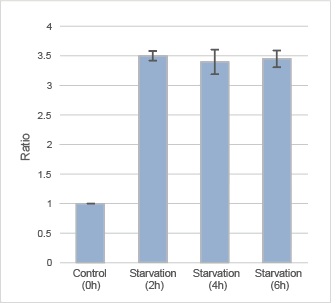
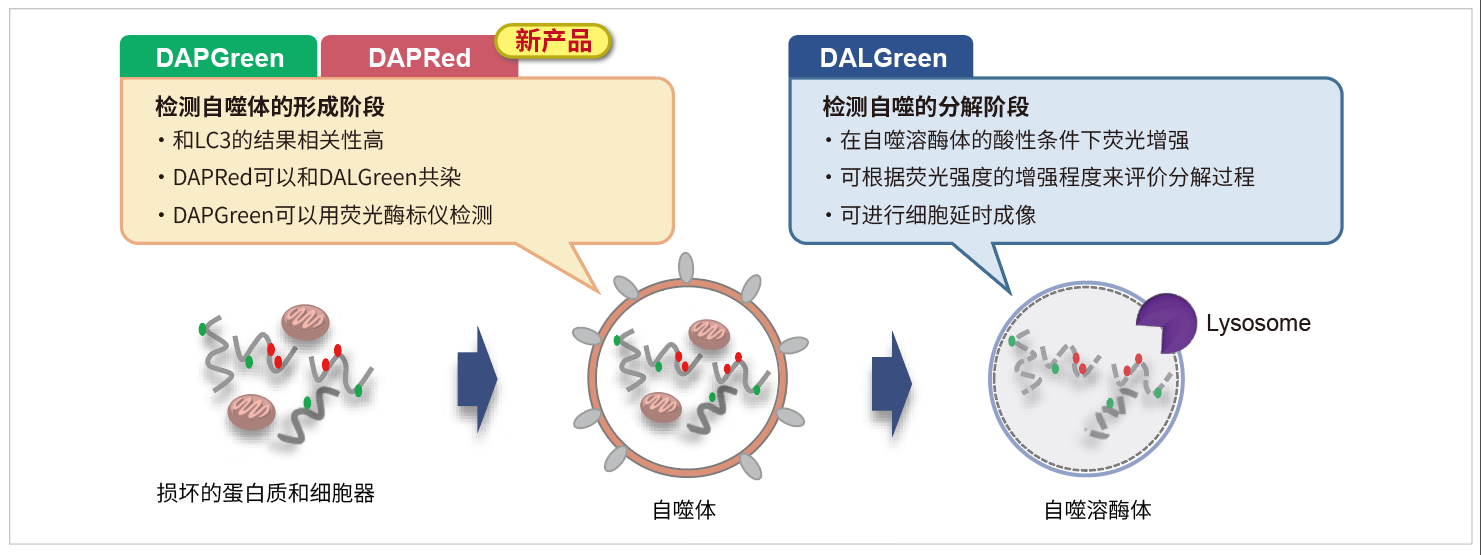
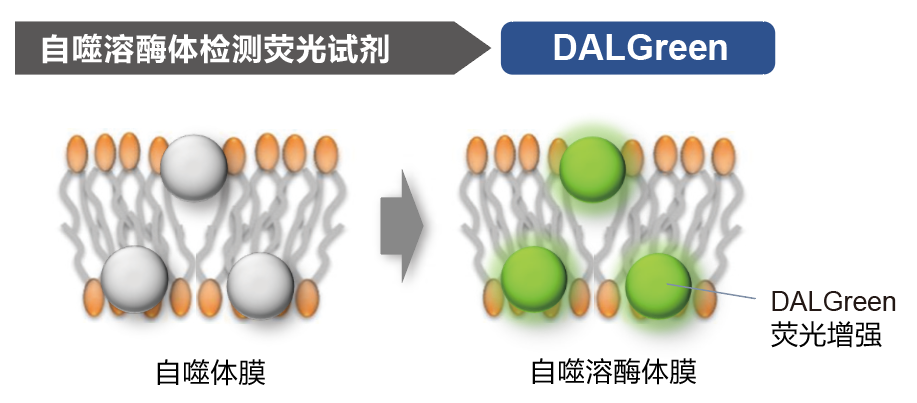
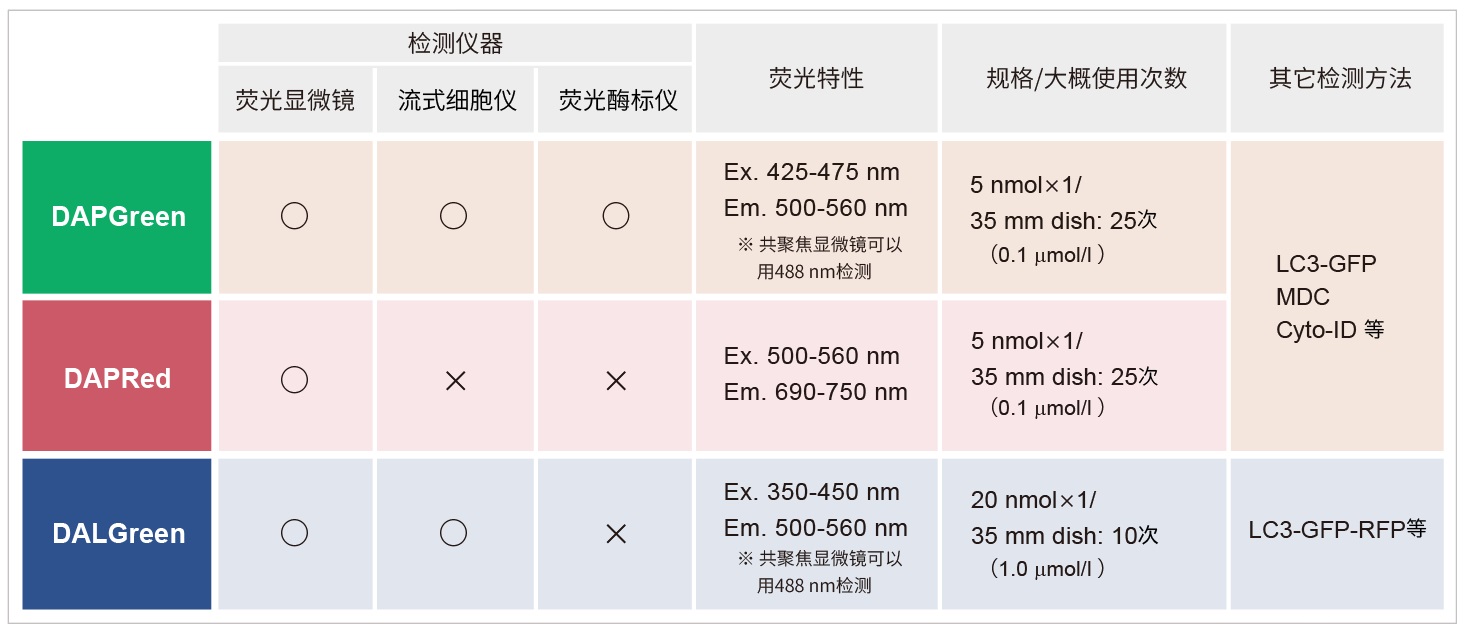
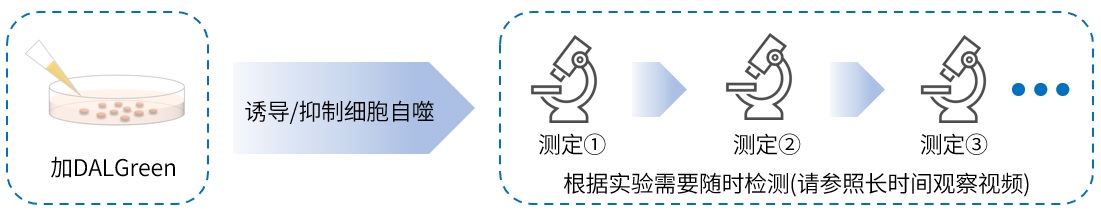
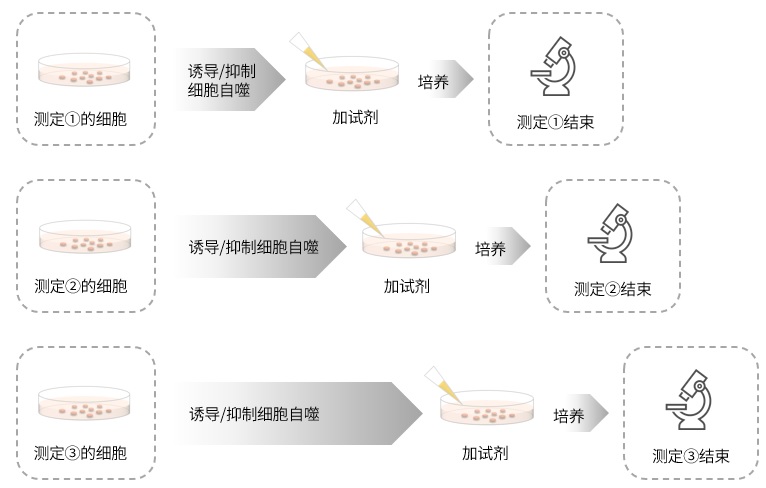
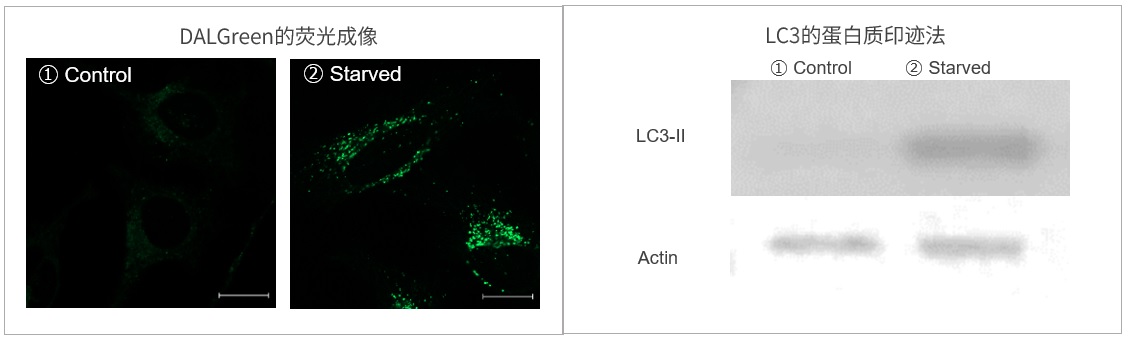
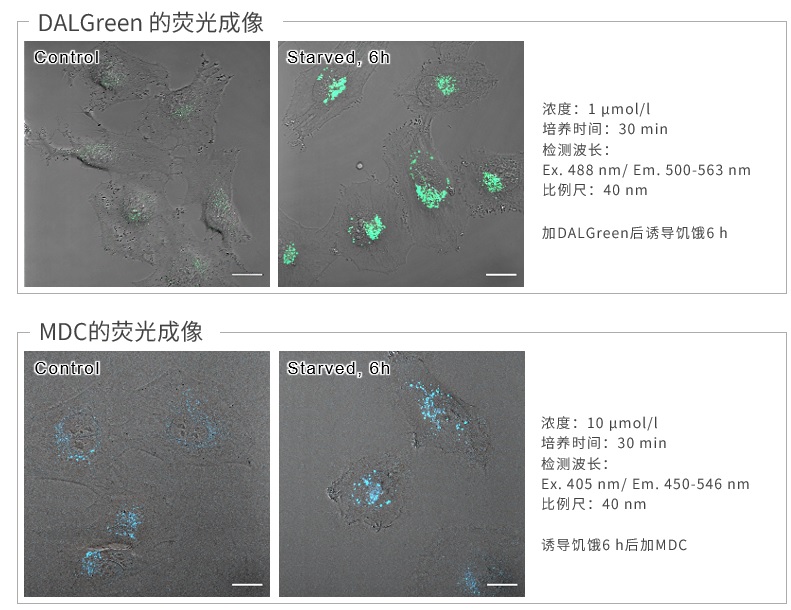
|
1.
|
A comparison of strategies for immortalizing mouse embryonic fibroblasts: M.M. St. Amad, et al.; J. Biol. Methods 3, e41 (2016), Application(s): Detected autophagy induction in SV40 transformed versus serially passed MEFs, 全文
|
|
2.
|
Astemizole-Histamine induces Beclin-1-independent autophagy by targeting p53-dependent crosstalk between autophagy and apoptosis: R. Jakhar, et al.; Cancer Lett. 372, 89 (2016), Application(s): Flow cytometry analysis, 摘要;
|
|
3.
|
Atg5 Is Essential for the Development and Survival of Innate Lymphocytes: T.E. O'Sullivan, et al.; Cell Rep. 15, 1910 (2016), Application(s): Liver lymphocytes were harvested and stained with cell surface antibodies and then incubated with 1:400 Cyto-ID Autophagy Detection Reagent, 摘要; 全文
|
|
4.
|
Autophagy-related gene 5 and Wnt5 signaling pathway requires differentiation of embryonic stem cells into odontoblast-like cells: N. Ozeki, et al.; Exp. Cell Res. 341, 92 (2016), Application(s): Autophagy flux, 摘要;
|
|
5.
|
DHA-induced stress response in human colon cancer cells-focus on oxidative stress and autophagy: K. Pettersen, et al.; Free Radic. Biol. Med. 90, 158 (2016), Application(s):Autophagy determined by flow cytometry, 摘要;
|
|
6.
|
Diosgenin induces ROS-dependent autophagy and cytotoxicity via mTOR signaling pathway in chronic myeloid leukemia cells: S. Jiang, et al.; Phytomedicine 23, 243 (2016),Application(s): Confocal immunofluorescence, 摘要;
|
|
7.
|
Efavirenz causes oxidative stress, endoplasmic reticulum stress, and autophagy in endothelial cells: M. Weiss, et al.; Cardiovasc. Toxicol. 16, 90 (2016), 摘要;
|
|
8.
|
MiR-193b promotes autophagy and non-apoptotic cell death in oesophageal cancer cells: M.J. Nyhan, et al.; BMC Cancer 16, 101 (2016), Application(s): Assay used to stain live cells, 摘要; 全文
|
|
9.
|
(Pro)renin receptor regulates autophagy and apoptosis in podocytes exposed to high glucose: C. Li, et al.; Am. J. Physiol. Endocrinol. Metab. 309, E302 (2015), Application(s):Confocal microscopy using mouse podocytes, 摘要;
|
|
10.
|
A rapid and high content assay that measures cyto-ID-stained autophagic compartments and estimates autophagy flux with potential clinical applications: S. Guo, et al.; Autophagy11, 560 (2015), Application(s): Fluorescent detection, Microplate Reader , 摘要; 全文
|
|
11.
|
A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal Erythropoiesis: R. Liang, et al.; PLoS Genet. 11, e1005526 (2015), Application(s):Autophagy flux measured by flow cytometry , 摘要; 全文
|
|
12.
|
ABT-888 enhances cytotoxic effects of temozolomide independent of MGMT status in serum free cultured glioma cells: R.K. Balvers, et al.; J. Transl. Med. 13, 74 (2015),Application(s): Assay, 摘要; 全文
|
|
13.
|
Activation of autophagy in response to nanosecond pulsed electric field exposure: J.C. Ullery, et al.; Biochem. Biophys. Res. Commun. 458, 411 (2015), Application(s):Fluorescence microscopy using U937 monocyte and CHO-K1 cell lines, 摘要;
|
|
14.
|
Aflatoxin biosynthesis is a novel source of reactive oxygen species-a potential redox signal to initiate resistance to oxidative stress?: L.V. Roze, et al.; Toxins (Basel). 7, 1411 (2015),Application(s): Assay, 摘要; 全文
|
|
15.
|
Alisertib induces cell cycle arrest and autophagy and suppresses epithelial-to-mesenchymal transition involving PI3K/Akt/mTOR and sirtuin 1-mediated signaling pathways in human pancreatic cancer cells: F. Wang, et al.; Drug Des. Devel. Ther. 9, 575 (2015), Application(s): Flow cytometry using PANC-1 and BxPC-3 pancreatic cancer cell lines, 摘要; 全文
|
|
16.
|
Alisertib, an Aurora kinase A inhibitor, induces apoptosis and autophagy but inhibits epithelial to mesenchymal transition in human epithelial ovarian cancer: Y.H. Ding, et al.; Drug Des. Devel. Ther. 9, 425 (2015), Application(s): Flow cytometry using SKOV3 and OVCAR-4 ovarian cancer cell lines, 摘要; 全文
|
|
17.
|
Andrographolide Analogue Induces Apoptosis and Autophagy Mediated Cell Death in U937 Cells by Inhibition of PI3K/Akt/mTOR Pathway: D. Kumar, et al.; PLoS One 10, e0139657 (2015), Application(s): Flow cytometric analysis of Cyto-ID Green Detection Reagent , 摘要; 全文
|
|
18.
|
Apoptotic Cell Death Induced by Resveratrol Is Partially Mediated by the Autophagy Pathway in Human Ovarian Cancer Cells: F. Lang, et al.; PLoS One 10, e0129196 (2015),Application(s): Live Cell Imaging, 摘要; 全文
|
|
19.
|
Araguspongine C induces autophagic death in breast cancer cells through suppression of c-Met and HER2 receptor tyrosine kinase signaling: M.R. Akl, et al.; Mar. Drugs 13, 288 (2015), Application(s): Flow cytometry using BT-474 breast cancer cell line, 摘要; 全文
|
|
20.
|
Autocrine VEGF maintains endothelial survival through regulation of metabolism and autophagy: C.K. Domigan, et al.; J. Cell. Sci. 128, 2236 (2015), 摘要;
|
|
21.
|
Autophagy is activated in systemic lupus erythematosus and required for plasmablast development: A.J. Clarke, et al.; Ann. Rheum. Dis. 74, 912 (2015), 摘要; 全文
|
|
22.
|
Autophagy limits proliferation and glycolytic metabolism in acute myeloid leukemia: A.S. Watson, et al.; Cell Death Discov. 1, 15008 (2015), Application(s): CytoID assay in human and mouse HSCs, 摘要; 全文
|
|
23.
|
Baicalin inhibits autophagy induced by influenza A virus H3N2: H.Y. Zhu, et al.; Antiviral Res. 113, 62 (2015), Application(s): Fluorescence microscopy using A549 human lung cancer cell line, 摘要;
|
|
24.
|
Bardoxolone methyl induces apoptosis and autophagy and inhibits epithelial-to-mesenchymal transition and stemness in esophageal squamous cancer cells: Y.Y. Wang, et al.; Drug Des. Devel. Ther. 9, 993 (2015), Application(s): Flow Cytometry, 摘要; 全文
|
|
25.
|
Cell-penetrating peptide derived from human eosinophil cationic protein inhibits mite allergen Der p 2 induced inflammasome activation: S.J. Yu, et al.; PLoS One 10, e0121393 (2015), Application(s): Flow cytometry of THP-1 leukemia cell line, 摘要; 全文
|
|
26.
|
Chemoproteomics Reveals Novel Protein and Lipid Kinase Targets of Clinical CDK4/6 Inhibitors in Lung Cancer: N.J. Sumi, et al.; ACS Chem. Biol. 10, 2680 (2015),Application(s): Quantification of autophagosomes, 摘要;
|
|
27.
|
Circulating hemocytes from larvae of the Japanese rhinoceros beetle Allomyrina dichotoma (Linnaeus) (Coleoptera: Scarabaeidae) and the cellular immune response to microorganisms: S. Hwang, et al.; PLoS One 10, e0128519 (2015), Application(s):Fluorescence microscopy using hemocytes from Japanese rhinoceros beetle Allomyrina dichotoma larvae, 摘要; 全文
|
|
28.
|
Citreoviridin induces ROS-dependent autophagic cell death in human liver HepG2 cells: Y.N. Liu, et al.; Toxicon. 95, 30 (2015), Application(s): Fluorescence microscopy using HepG2 cell line, 摘要;
|
|
29.
|
Clozapine induces autophagic cell death in non-small cell lung cancer cells: Y.C. Yin, et al.; Cell. Physiol. Biochem. 35, 945 (2015), 摘要;
|
|
30.
|
Coffee and caffeine potentiate the antiamyloidogenic activity of melatonin via inhibition of Aβ oligomerization and modulation of the Tau-mediated pathway in N2a/APP cells: L.F. Zhang, et al.; Drug Des. Devel. Ther. 9, 241 (2015), Application(s): Flow Cytometry,摘要; 全文
|
|
31.
|
Combination of the mTOR inhibitor RAD001 with temozolomide and radiation effectively inhibits the growth of glioblastoma cells in culture: H. Burckel, et al.; Oncol. Rep. 33, 471 (2015), 摘要;
|
|
32.
|
Danusertib Induces Apoptosis, Cell Cycle Arrest, and Autophagy but Inhibits Epithelial to Mesenchymal Transition Involving PI3K/Akt/mTOR Signaling Pathway in Human Ovarian Cancer Cells: D. Zi, et al.; Int. J. Mol. Sci. 16, 27228 (2015), Application(s): Confocal fluorescence microscopy, 摘要; 全文
|
|
33.
|
Danusertib, a potent pan-Aurora kinase and ABL kinase inhibitor, induces cell cycle arrest and programmed cell death and inhibits epithelial to mesenchymal transition involving the PI3K/Akt/mTOR-mediated signaling pathway in human gastric cancer AGS and NCI-N78 cells: C.X. Yuan, et al.; Drug Des. Devel. Ther. 9, 1293 (2015), Application(s): Flow cytometry using AGS and NCI-N78 gastric cancer cell lines, 摘要; 全文
|
|
34.
|
Defective autophagy in vascular smooth muscle cells alters contractility and Ca²⁺ homeostasis in mice: C.F. Michiels, et al.; Am. J. Physiol. Heart Circ. Physiol. 308, H557 (2015), 摘要;
|
|
35.
|
Effects of cyclodextrins on GM1‐gangliosides in fibroblasts from GM1‐gangliosidosis patients: Y. Maeda, et al.; J. Pharm. Pharmacol. 67, 1133 (2015), 摘要;
|
|
36.
|
Endurance exercise training induces fat depot-specific differences in basal autophagic activity: G. Tanaka, et al.; Biochem. Biophys. Res. Commun. 466, 512 (2015),Application(s): Detect the formation of autophagosomes, 摘要;
|
|
37.
|
Erbin is a novel substrate of the Sag-βTrCP E3 ligase that regulates KrasG12D-induced skin tumorigenesis: C.M. Xie, et al.; J. Cell. Biol. 209, 721 (2015), 摘要;
|
|
38.
|
Evaluation of Antitumor Effects of Folate-Conjugated Methyl-β-cyclodextrin in Melanoma: K. Motoyama, et al.; Biol. Pharm. Bull. 38, 374 (2015), Application(s): Fluorescence Microscopy, 摘要; 全文
|
|
39.
|
Exchange protein directly activated by cAMP 1 promotes autophagy during cardiomyocyte hypertrophy: A.C. Laurent, et al.; Cardiovasc. Res. 105, 55 (2015), Application(s):Fluorescence microscopy using rat neonatal ventricular myocytes, 摘要;
|
|
40.
|
Glutathione-S-transferase omega 1 (GSTO1-1) acts as mediator of signaling pathways involved in aflatoxin B1-induced apoptosis-autophagy crosstalk in macrophages: S. Paul, et al.; Free Radic. Biol. Med. 89, 1218 (2015), Application(s): Determination of autophagy with immunocytochemistry , 摘要;
|
|
41.
|
GMI, an immunomodulatory protein from Ganoderma microsporum, potentiates cisplatin-induced apoptosis via autophagy in lung cancer cells: I.L. Hsin, et al.; Mol. Pharm. 12, 1534 (2015), 摘要;
|
|
42.
|
Induction of apoptosis and autophagy via sirtuin1- and PI3K/Akt/mTOR-mediated pathways by plumbagin in human prostate cancer cells: Z.W. Zhou, et al.; Drug Des. Devel. Ther. 9, 1511 (2015), Application(s): Assay, 摘要; 全文
|
|
43.
|
Induction of autophagy is a key component of all-trans-retinoic acid-induced differentiation in leukemia cells and a potential target for pharmacologic modulation: N. Orfali, et al.; Exp. Hematol. 43, 781 (2015), Application(s): Flow cytometry analysis of NB4 and HL60 promyelocytic leukemia cell lines, 摘要;
|
|
44.
|
Inhibition of Autophagy Potentiated the Antitumor Effect of Nedaplatin in Cisplatin-Resistant Nasopharyngeal Carcinoma Cells: Z. Liu, et al. ; PLoS One 10, e0135236 (2015),Application(s): Cell culture, 摘要; 全文
|
|
45.
|
Inhibition of mitotic Aurora kinase A by alisertib induces apoptosis and autophagy of human gastric cancer AGS and NCI-N78 cells: C.X. Yuan, et al.; Drug Des. Devel. Ther. 9, 487 (2015), Application(s): Flow cytometry using AGS and NCI-N78 gastric cancer cell lines, 摘要; 全文
|
|
46.
|
Interferon Regulatory Factor-1 signaling regulates the switch between autophagy and apoptosis to determine breast cancer cell fate: J.L. Schwartz-Roberts, et al.; Cancer Res.75, 1046 (2015), 摘要;
|
|
47.
|
Interplay of Oxidative Stress and Autophagy in PAMAM Dendrimers-Induced Neuronal Cell Death : Y. Li, et al.; Theranostics 5, 1363 (2015), Application(s): Confocal fluorescence assay, 摘要; 全文
|
|
48.
|
Invariant NKT cells require autophagy to coordinate proliferation and survival signals during differentiation: B. Pei, et al.; J. Immunol. 194, 5872 (2015), 摘要;
|
|
49.
|
Involvement of fish signal transducer and activator of transcription 3 (STAT3) in nodavirus infection induced cell death: Y. Huang, et al.; Fish Shellfish Immunol. 43, 241 (2015),Application(s): Fluorescence microscopy of Grouper (fish) brain cells, 摘要;
|
|
50.
|
Is the autophagy a friend or foe in the silver nanoparticles associated radiotherapy for glioma?: H. Wu, et al.; Biomaterials 62, 47 (2015), Application(s): Fluorescence microscopy using U251 human glioma cell line, 摘要;
|
|
51.
|
Kaposi's sarcoma-associated herpesvirus induces Nrf2 activation in latently infected endothelial cells through SQSTM1 phosphorylation and interaction with polyubiquitinated Keap1: O. Gjyshi, et al.; J. Virol. 89, 2268 (2015), 摘要;
|
|
52.
|
KLF4-SQSTM1/p62-associated prosurvival autophagy contributes to carfilzomib resistance in multiple myeloma models: I. Riz, et al.; Oncotarget 6, 17814 (2015), Application(s):FACS, 摘要; 全文
|
|
53.
|
Lithium modulates autophagy in esophageal and colorectal cancer cells and enhances the efficacy of therapeutic agents in vitro and in vivo: T.R. O'Donovan, et al.; PLoS One 10, e0134676 (2015), Application(s): Flow cytometry analysis using human esophageal and murine colon cancer cell lines, 摘要; 全文
|
|
54.
|
Methicillin-Resistant Staphylococcus aureus Adaptation to Human Keratinocytes: G. Soong, et al.; MBio. 6, e00289-15 (2015), Application(s): Assay, 摘要; 全文
|
|
55.
|
Mevalonate pathway regulates cell size homeostasis and proteostasis through autophagy: T.P. Miettinen, et al.; Cell Rep. 13, 2610 (2015), Application(s): Flow cytometry analysis of autophagy using Jurkat, U2OS, Kc167 and HUVEC cells, 摘要;
|
|
56.
|
MiR-29b replacement inhibits proteasomes and disrupts aggresome+autophagosome formation to enhance the antimyeloma benefit of bortezomib: S. Jagannathan, et al.; Leukemia 29, 727 (2015), Application(s): Detection of autophagy by fluorescence microscopy in multiple myeloma cell lines, 摘要; 全文
|
|
57.
|
Molecular chaperone GRP78 enhances aggresome delivery to autophagosomes to promote drug resistance in multiple myeloma: M.A. Abdel Malek, et al.; Oncotarget 6, 3098 (2015), Application(s): Confocal Microscopy, 摘要; 全文
|
|
58.
|
Molecular cloning and characterization of autophagy-related gene TmATG8 in Listeria-invaded hemocytes of Tenebrio molitor: H. Tindwa, et al.; Dev. Comp. Immunol. 51, 88 (2015), Application(s): Fluorescence microscopy using hemocytes from Tenebrio molitor larvae, 摘要;
|
|
59.
|
Molecular pathway of near-infrared laser phototoxicity involves ATF-4 orchestrated ER stress: I. Khan, et al.; Sci. Rep. 5, 10581 (2015), Application(s): Fluorescence microscopy autophagy assay, 摘要; 全文
|
|
60.
|
N-Myc and STAT Interactor regulates autophagy and chemosensitivity in breast cancer cells: B.J. Metge, et al.; Sci. Rep. 5, 11995 (2015), Application(s): Fluorescent detection,摘要; 全文
|
|
61.
|
Novel autophagy inducers lentztrehaloses A, B and C: S.I. Wada, et al.; J. Antibiot. (Tokyo)68, 521 (2015), Application(s): Fluorescence microscopy using Mewo melanoma and OVK18 ovarian cancer cell lines, 摘要;
|
|
62.
|
Novel small-molecule SIRT1 inhibitors induce cell death in adult T-cell leukaemia cells: T. Kozako, et al.; Sci. Rep. 5, 11345 (2015), Application(s): Flow cytometry using a variety of cancer cell lines, 摘要; 全文
|
|
63.
|
Novel targeting of PEGylated liposomes for codelivery of TGF-β1 siRNA and four antitubercular drugs to human macrophages for the treatment of mycobacterial infection: a quantitative proteomic study: N. Niu, et al. ; Drug Des. Devel. Ther. 9, 4441 (2015),Application(s): Autophagy of human macrophages by flow cytometry, 摘要;
|
|
64.
|
Paraptosis cell death induction by the thiamine analog benfotiamine in leukemia cells: N. Sugimori, et al.; PLoS One 10, e0120709 (2015), Application(s): Flow cytometry using HL60 leukemia cell line, 摘要; 全文
|
|
65.
|
Plumbagin induces G2/M arrest, apoptosis, and autophagy via p38 MAPK- and PI3K/Akt/mTOR-mediated pathways in human tongue squamous cell carcinoma cells: S.T. Pan, et al.; Drug Des. Devel. Ther. 9, 1601 (2015), Application(s): Assay, 摘要; 全文
|
|
66.
|
Pro-apoptotic and pro-autophagic effects of the Aurora kinase A inhibitor alisertib (MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the activation of mitochondria-mediated pathway and inhibition of p38 MAPK/PI3K/Akt/mTOR signaling pathway: N.K. Niu, et al.; Drug Des. Devel. Ther. 9, 1555 (2015), Application(s): Assay,摘要; 全文
|
|
67.
|
Reduced FoxO3a expression causes low autophagy in idiopathic pulmonary fibrosis fibroblasts on collagen matrix: J. Im, et al.; Am. J. Physiol. Lung Cell. Mol. Physiol. 309, L552 (2015), 摘要;
|
|
68.
|
S-Adenosyl-L-methionine-competitive inhibitors of the histone methyltransferase EZH2 induce autophagy and enhance drug sensitivity in cancer cells: T.P. Liu, et al.; Anticancer Drugs 26, 139 (2015), Application(s): Fluorescence microscopy using MDA-MB-231 breast cancer cell line, 摘要; 全文
|
|
69.
|
Schisandrin B inhibits cell growth and induces cellular apoptosis and autophagy in mouse hepatocytes and macrophages: implications for its hepatotoxicity: Y. Zhang, et al.; Drug Des. Devel. Ther. 9, 2001 (2015), Application(s): Flow cytometry using AML-12 hepatocyte and RAW 264.7 leukemia cell lines, 摘要; 全文
|
|
70.
|
Src/STAT3-dependent heme oxygenase-1 induction mediates chemoresistance of breast cancer cells to doxorubicin by promoting autophagy: Q. Tan, et al.; Cancer Sci. 106, 1023 (2015), 摘要;
|
|
71.
|
The CCL2 chemokine is a negative regulator of autophagy and necrosis in luminal B breast cancer cells: W.B. Fang, et al.; Breast Cancer Res. Treat. 150, 309 (2015), 摘要;
|
|
72.
|
The investigational Aurora kinase A inhibitor alisertib (MLN8237) induces cell cycle G2/M arrest, apoptosis, and autophagy via p38 MAPK and Akt/mTOR signaling pathways in human breast cancer cells: J.P. Li, et al.; Drug Des. Devel. Ther. 9, 1627 (2015),Application(s): Assay, 摘要; 全文
|
|
73.
|
The pan-inhibitor of Aurora kinases danusertib induces apoptosis and autophagy and suppresses epithelial-to-mesenchymal transition in human breast cancer cells: J.P. Li, et al.; Drug Des. Devel. Ther. 9, 1027 (2015), Application(s): Assay, 摘要; 全文
|
|
74.
|
The role of autophagy in the cytotoxicity induced by recombinant human arginase in laryngeal squamous cell carcinoma: C. Lin, et al.; Appl. Microbiol. Biotechnol. 99, 8487 (2015), 摘要;
|
|
75.
|
A Novel CXCR3-B Chemokine Receptor-induced Growth-inhibitory Signal in Cancer Cells Is Mediated through the Regulation of Bach-1 Protein and Nrf2 Protein Nuclear Translocation : M. Balan & S. Pal; J. Biol. Chem. 289, 3126 (2014), Application(s): Monitor autophagy in MCF-7 and T47D breast cancer cells by flow cytometry and fluorescence microscopy, 摘要;
|
|
76.
|
Adaptive responses to glucose restriction enhance cell survival, antioxidant capability, and autophagy of the protozoan parasite Trichomonas vaginalis: K.Y. Huang, et al.; Biochim. Biophys. Acta. 1840, 53 (2014), 摘要;
|
|
77.
|
Autophagy in the brain of neonates following hypoxia-ischemia shows sex-and region-specific effects: S.N. Weis, et al.; Neuroscience 256, 201 (2014), 摘要;
|
|
78.
|
Cannabinoid-induced autophagy regulates suppressor of cytokine signaling-3 in intestinal epithelium: L.C. Koay, et al.; Am. J. Physiol. Gastrointest. Liver Physiol. 307, G140 (2014),Application(s): Detection of autophagy in human colonic epithelial cell line Caco-2 by Confocal imaging, 摘要; 全文
|
|
79.
|
Caveolin-1 Is a Critical Determinant of Autophagy, Metabolic Switching, and Oxidative Stress in Vascular Endothelium: T. Shiroto, et al.; PLoS One 9, e87871 (2014), 摘要;全文
|
|
80.
|
Connective tissue diseases: How do autoreactive B cells survive in SLE-autophagy?: N.J. Bernard; Nat. Rev. Rheumatol. 10, 128 (2014), (Review), 摘要;
|
|
81.
|
Defective Autophagosome Trafficking Contributes to Impaired Autophagic Flux in Coronary Arterial Myocytes Lacking CD38 Gene: Y. Zhang, et al.; Cardiovasc. Res. 102, 68 (2014),摘要;
|
|
82.
|
Defects in mitochondrial clearance predispose human monocytes to interleukin-1β hyper-secretion: R. van der Burgh, et al.; J. Biol. Chem. 289, 5000 (2014), 摘要; 全文
|
|
83.
|
Early biomarkers of response to carfilzomib in multiple myeloma (MM): Modulation of CXCR4 and induction of autophagy: M. Bhutani, et al.; J. Clin. Oncol. 32, e19572 (2014),Application(s): Quantification of autophagy in malignant plasma cells from bone marrow aspirates by flow cytometry with the Cyto-ID autophagy detection kit,
|
|
84.
|
Enhancement of dynein-mediated autophagosome trafficking and autophagy maturation by ROS in mouse coronary arterial myocytes: M. Xu, et al.; J. Cell. Mol. Med. 18, 2165 (2014), 摘要; 全文
|
|
85.
|
Flow Cytometric Analysis of Autophagic Activity with Cyto-ID Staining in Primary Cells: M. Stankov, et al.; Bio-Protocol (2014), Application(s): FC in primary BMDCs, 全文
|
|
86.
|
High-Content Assays for Hepatotoxicity Using Induced Pluripotent Stem Cell-Derived Cells: O. Sirenko, et al.; Assay Drug Dev. Technol. 12, 43 (2014), 摘要; 全文
|
|
87.
|
Histone deacetylase inhibitors induce apoptosis in myeloid leukemia by suppressing autophagy: M.V. Stankov, et al.; Leukemia 28, 577 (2014), 摘要;
|
|
88.
|
Histone deacetylase inhibitors potentiate VSV oncolysis in prostate cancer cells by modulating NF-κB dependent autophagy: L. Shulak, et al.; J. Virol. 88, 2927 (2014),摘要;
|
|
89.
|
In vitro and in vivo characterization of porcine acellular dermal matrix for gingival augmentation procedures: A.M. Pabst, et al.; J. Periodontal. Res. 49, 371 (2014), 摘要;
|
|
90.
|
Inhibition of Autophagic Flux by Salinomycin Results in Anti-Cancer Effect in Hepatocellular Carcinoma Cells: J. Klose, et al.; PLoS One 9, e95970 (2014),Application(s): Autophagy detection in human hepatocellular carcinoma , 摘要; 全文
|
|
91.
|
Inhibition of stress induced premature senescence in presenilin-1 mutated cells with water soluble Coenzyme Q10: D. Ma, et al.; Mitochondrion 17C, 106 (2014), Application(s):Autophagic vacuoles in Alzheimer's Disease fibroblasts detected with CytoID® Green Autophagy Detection kit, 摘要;
|
|
92.
|
Involvement of autophagy in recombinant human arginase-induced cell apoptosis and growth inhibition of malignant melanoma cells: Z. Wang, et al.; Appl. Microbiol. Biotechnol.98, 2485 (2014), 摘要;
|
|
93.
|
MiR-216a: a link between endothelial dysfunction and autophagy: R. Menghini, et al.; Cell Death Dis. 5, e1029 (2014), 摘要;
|
|
94.
|
Novel estradiol analogue induces apoptosis and autophagy in esophageal carcinoma cells: E. Wolmarans, et al.; Cell. Mol. Biol. Lett. 19 , 98 (2014), Application(s): Autophagy detection in esophageal carcinoma SNO cell , 摘要;
|
|
95.
|
Novel sorafenib-based structural analogues: in-vitro anticancer evaluation of t-MTUCB and t-AUCMB: A.T. Wecksler, et al.; Anticancer Drugs 25, 433 (2014), 摘要;
|
|
96.
|
Photodynamic therapy with the novel photosensitizer chlorophyllin f induces apoptosis and autophagy in human bladder cancer cells: D. Lihuan, et al.; Lasers Surg. Med. 46, 319 (2014), 摘要;
|
|
97.
|
Plumbagin induces apoptotic and autophagic cell death through inhibition of the PI3K/Akt/mTOR pathway in human non-small cell lung cancer cells: Y.C.Li, et al.; Cancer Lett. 344, 239 (2014), 摘要;
|
|
98.
|
Potential of adenovirus-mediated REIC/Dkk-3 gene therapy for use in the treatment of pancreatic cancer: D. Uchida, et al.; J. Gastroenterol. Hepatol. 29, 973 (2014), 摘要;
|
|
99.
|
Sirt1 modulates endoplasmic reticulum stress-induced autophagy in heart: A. Guilbert, et al.; Cardiovasc. Res. 103 (suppl 1), S13 (2014), Application(s): Evaluation of Autophagy in H9c2 cells, rat cardiomyoblasts by flow cytometry, 全文
|
|
100.
|
STAT3 down regulates LC3 to inhibit autophagy and pancreatic cancer cell growth: J. Gong, et al.; Oncotarget 5, 2529 (2014), Application(s): Autophagic vacuole formation was detected by microscopy and autophagosome formation was determined by flow cytometry in human pancreatic cancer cells Capan-2, 摘要; 全文
|


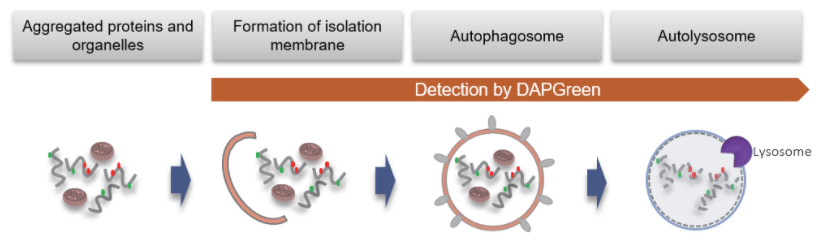
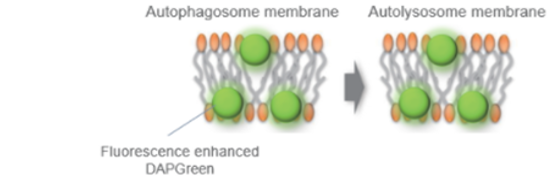
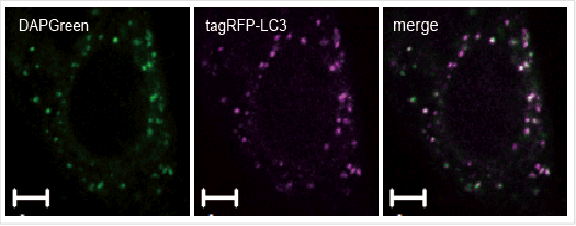
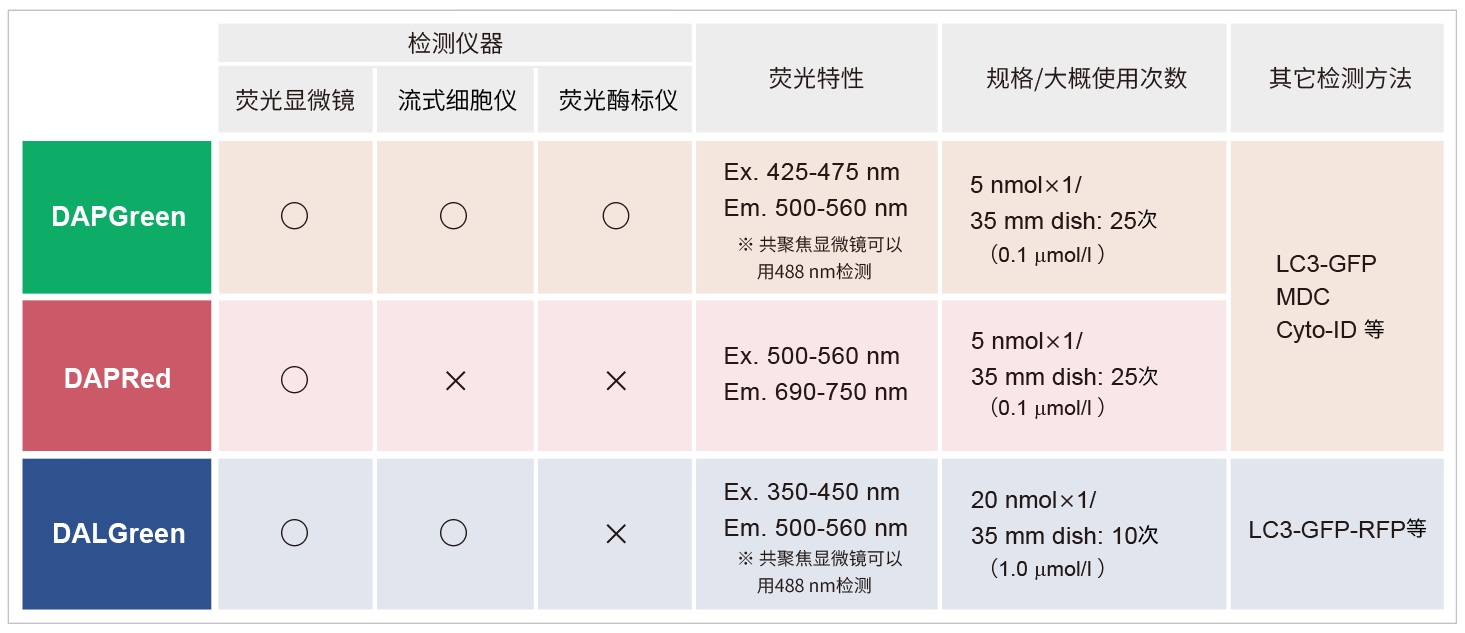 *DAPGreen和DALGreen不能共染
*DAPGreen和DALGreen不能共染