上海金畔生物科技有限公司代理日本同仁化学 DOJINDO代理商全线产品,欢迎访问官网了解更多信息
特点:
● 灵敏度高
● 易上手
● 多种仪器均可检测


产品解说
活动进行中
订购满5000元,200元礼品等你拿
凑单关联产品TOP5
NO.1. Cell Counting Kit-8 细胞增殖毒性检测
NO.2. ROS Assay Kit 活性氧检测
NO.3. FerroOrange 细胞亚铁离子检测
NO.4. GSSG/GSH Quantification Kit II 氧化型/还原型谷胱甘肽
NO.5. Mitophagy Detection Kit 线粒体自噬检测
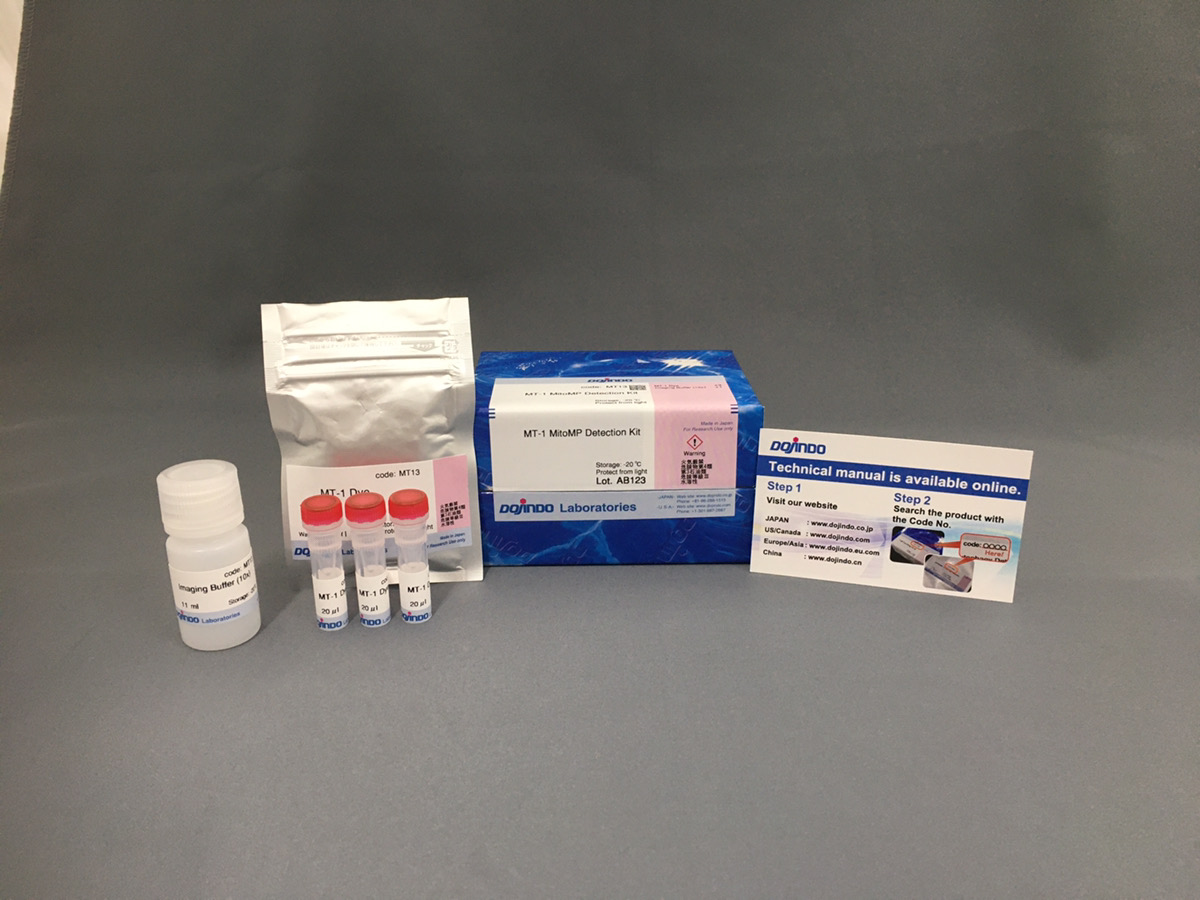
试剂盒内含

产品概述
细胞中的线粒体作为有氧呼吸产生ATP的主要场所,是体内重要的细胞器之一,常被用于早期细胞毒性、氧化应激、细胞凋亡等研究中1)。线粒体活性的降低与机能失调,已被证实与癌症、衰老、神经退行性疾病 (如阿尔兹海默症、帕金森病等) 等密切相关2)3)。
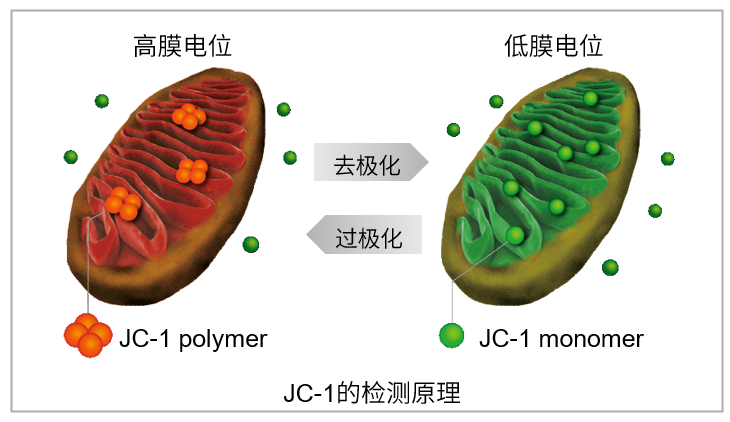
JC-1是一种被广泛使用的小分子线粒体膜电位探针,依赖于线粒体膜电位在线粒体中聚集,染料伴随聚集过程,荧光从绿色 (530 nm) 变为红色 (590 nm)。当线粒体发生去极化,红/绿荧光强度比值降低。以往的研究者反映,JC-1不易溶于水并有大量沉淀产生。但与其他公司的产品不同,同仁化学研究所研制的JC-1试剂解决了这一问题,避免了沉淀的产生。同时使用试剂盒中配制的成像缓冲液 (Imaging Buffer),可大幅降低荧光背景并在检测过程中保护细胞不受损伤。
当JC-1工作液的浓度为2 μmol/l, 每次用量为100 μl时,可以检测500次。
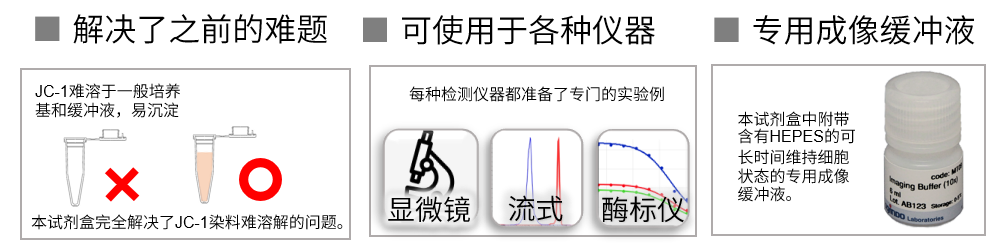
产品特点
1.为什么要检测线粒体膜电位
线粒体不仅是细胞内产生能量的场所,它还与癌症、衰老、阿尔兹海默症、帕金森等神经变异性疾病密切相关。因此,针对线粒体状态的研究非常重要,其中线粒体膜电位的变化经常被作为重要的指标之一检测。
当线粒体正常、膜电位差保持不变时,JC-1会聚集并发出红色荧光,而当膜电位降低时,JC-1会作为单体存在并发出绿色荧光。红色和绿色荧光强度的变化可以作为检测线粒体状态的指标。
2.初次使用也很容易上手
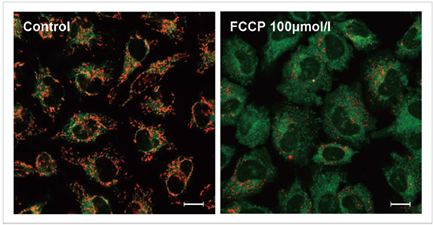
3.去极化的检测实例
使用去极化剂carbonylcyanide-p-trifluoromethoxyphenylhydrazone(FCCP)对HeLa细胞进行处理,用本试
剂盒进行检测。可以发现与未加药物的细胞相比,加药组细胞的红色荧光明显减少。
实验条件
JC-1浓度: 2 μmol/l in MEM, 染色时间30 min
FCCP浓度:100 μmol/l, FCCP处理时间1 h
检测条件
Green : Ex 488 nm/ Em 500-550 nm;
Red : Ex 561 nm/ Em 560-610 nm;
标尺: 20 μm
操作步骤
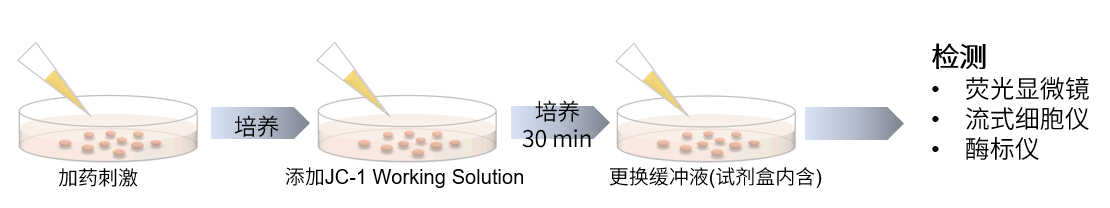
实验例
1.诱导凋亡的实验例
1.1 荧光显微镜
通过荧光颜色的改变判断由凋亡导致的线粒体膜电位的变化。
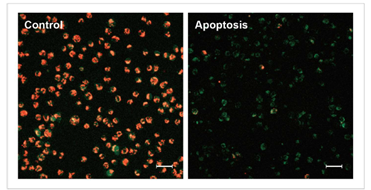
检测条件
Green: Ex 488 nm / Em 500-550 nm
Red : Ex 561 nm / Em 560-610 nm
标尺: 80 μm
1.2 流式细胞仪
定量分析单个细胞的膜电位变化
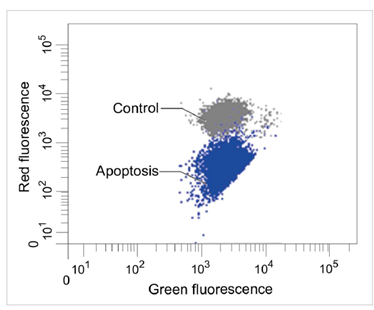
检测条件
Green: Ex 488 nm / Em 515-545 nm
Red : Ex 488 nm / Em 564-604 nm
1.3 酶标仪
确认孔板中吸光度来判断线粒体膜电位的变化
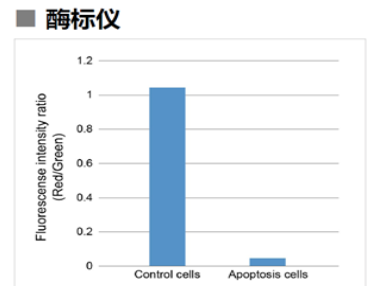
检测条件
Green: Ex 485 nm / Em 525-545 nm
Red : Ex 535 nm / Em 585-605 nm
2.诱导自噬的实验例
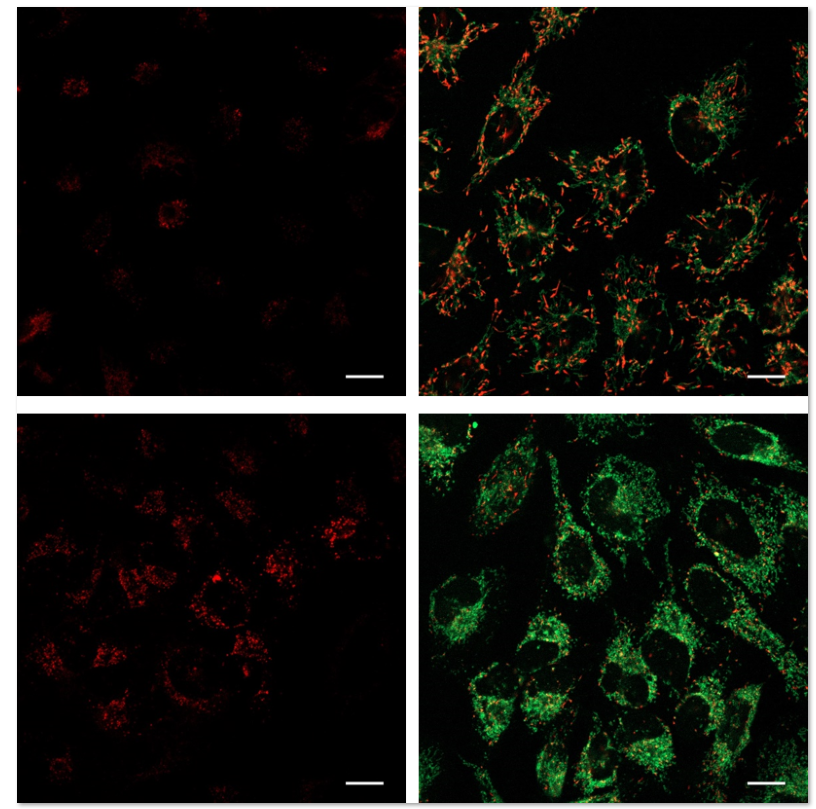
使用表达Parkin的HeLa细胞,分别使用线粒体自噬试剂盒(Mitophagy Detection Kit:MD01)和线粒体膜电位检测试剂盒(JC-1 MitoMP Detection Kit: MT09)来观察添加和不添加CCCP(羰基氰化物间氯苯)的线粒体状态的变化。
结果证明在未经CCCP处理的细胞中几乎未检测到线粒体自噬的发生,并且线粒体膜电位正常维持。 而在添加了CCCP的细胞中,证实了线粒体膜电位的降低(JC-1的红色荧光的降低)和线粒体的自噬(Mtphagy染料的荧光的增强)。
<检测条件>
线粒体自噬检测
Ex:561 nm,Em:570-700 nm
线粒体膜电位检测
绿色Ex:488 nm,Em:500-550 nm
红色Ex:561 nm,Em:560-610 nm
实验条件
1.将Parkin质粒导入HeLa细胞
使用HilyMax(货号:H357)将Parkin质粒引入HeLa细胞中(Parkin质粒/HilyMax试剂:0.1 μg/0.2 μl)
然后过夜培养,收集细胞进行以下检测。
2.自噬检测
向表达Parkin的HeLa细胞中添加0.1 μmol/l Mtphagy工作溶液,并在37°C下孵育30分钟。然后将细胞用HBSS洗涤,加入10 μg/ml CCCP/MEM溶液,并在37℃下孵育2小时。荧光显微镜下观察处理后的细胞。
3.线粒体膜电位检测
将10 μg/ml的CCCP/MEM溶液添加至表达Parkin的HeLa细胞中,并在37℃下孵育1.5小时。加入4 μmol/l的JC-1工作溶液使终浓度至2 μmol/l,并将细胞溶液在37℃下孵育30分钟。孵育后将细胞用HBSS洗涤,加入成像缓冲液,在荧光显微镜下观察细胞。
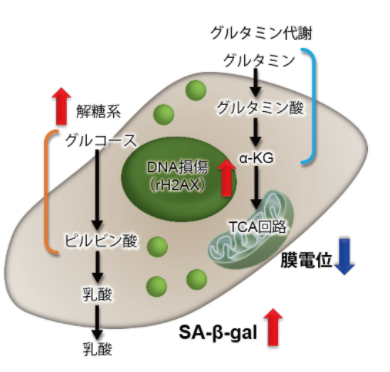
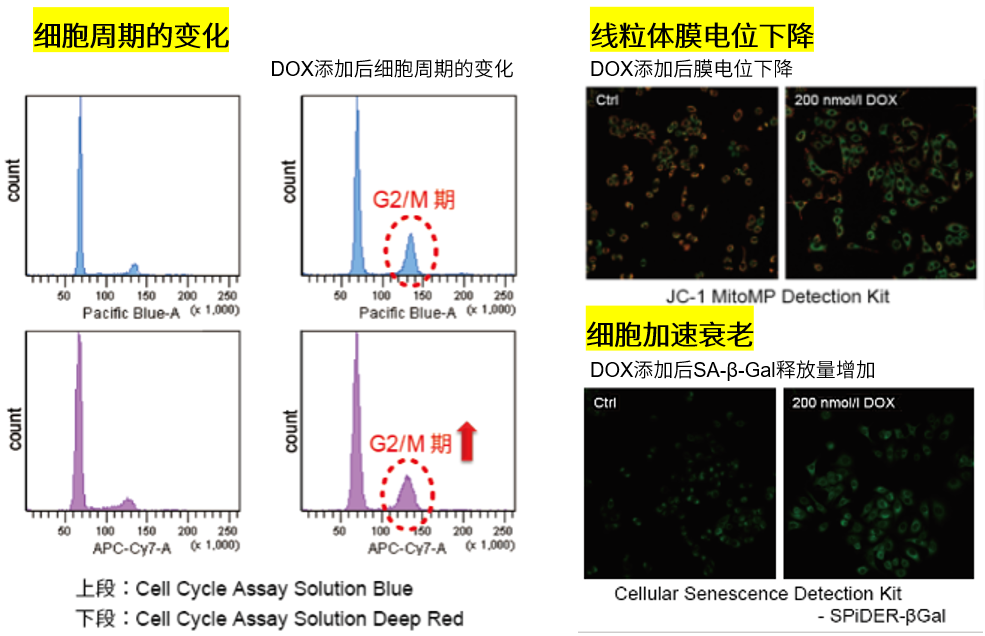
3.线粒体膜电位与细胞周期关联性
将已知能在细胞周期的G2/M期起作用以终止细胞增殖并诱导细胞衰老的阿霉素(DOX)加入A549细胞后,
使用细胞周期检测试剂盒蓝色(产品代码:C549)/深红色(产品代码:C548)后检测。
结果证实了A549细胞的细胞周期确实发生了变化,同时用细胞衰老检测试剂盒–SPiDER-βGal(产品代码:SG03)证实了细胞产生衰老,实验证实了线粒体膜电位会发生变化。
参考文献
| No. | Sample Type | Instrument | Reference |
| 1 | Cell:A549 | Microscope | K. Li, S. Sun, L. Xiao and Z. Zhang, “Bioactivity-guided fractionation of Helicteres angustifolia L. extract and its molecular evidence for tumor suppression”, Front Cell Dev Biol.,2023, doi: 10.3389/fcell.2023.1157172. |
| 2 | Cell:A549 | Flow Cytometer | C. N. D’Alessandro-Gabazza, T. Yasuma, T. Kobayashi, M. Toda1, A. M. Abdel-Hamid, H. Fujimoto, O. Hataji, H. Nakahara, A. Takeshita, K. Nishihama, T. Okano, H. Saiki, Y. Okano, A. Tomaru, V. F. D’Alessandro, M. Shiraishi, A. Mizoguchi, R. Ono, J. Ohtsuka, M. Fukumura, T. Nosaka, X. Mi, D. Shukla, K. Kataoka, Y. Kondoh, M. Hirose, T. Arai, Y. Inoue, Y. Yano, R. I. Mackie, I. Cann and E. C. Gabazza, “Inhibition of lung microbiota-derived proapoptotic peptides ameliorates acute exacerbation of pulmonary fibrosis”, Nat. Comm., 2022, doi:10.1038/s41467-022-29064-3. |
| 3 | Cell:A549, HeLa | Plate reader | J. Yang, L. Liu, Y. Oda, K. Wada, M. Ago, S. Matsuda, M. Hattori, T. Goto, Y. Kawashima, Y. Matsuzaki and T. Taketani,”Highly-purified rapidly expanding clones, RECs, are superior for functional-mitochondrial transfer”, Stem Cell Res Ther., 2023, doi: 10.1186/s13287-023-03274-y. |
| 4 | Cell:ALM | Plate reader | T. Nechiporuk, S.E. Kurtz, O. Nikolova, T. Liu, C.L. Jones, A. D. Alessandro, R. C. Hill, A. Almeida, S. K. Joshi, M. Rosenberg, C. E. Tognon, A. V. Danilov, B. J. Druker, B. H. Chang, S. K McWeeney and J. W. Tyner , “The TP53 Apoptotic Network Is a Primary Mediator of Resistance to BCL2 Inhibition in AML Cells.”, Cancer Discov, 2019, 9, |
| 5 | Cell:ARPE-19 | Flow Cytometer/ | J. Hamuro, T. Yamashita, Y. Otsuki, N. Hiramoto, M. Adachi, T. Miyatani, H. Tanaka, M. Ueno, S. Kinoshita and C. Sotozono,”Spatiotemporal Coordination of RPE Cell Quality by Extracellular Vesicle miR-494-3p Via Competitive Interplays With SIRT3 or PTEN”, Invest Ophthalmol Vis Sci., 2023, doi: 10.1167/iovs.64.5.9. |
| 6 | Cell:ARPE-19 | Microscope | J. H. Quan, F. F. Gao, H. A. Ismail, J. M. Yuk, G. H. Cha, J. Q. Chu and Y. H. Lee, “Silver Nanoparticle-Induced Apoptosis in ARPE-19 Cells Is Inhibited by Toxoplasma gondii Pre-Infection Through Suppression of NOX4-Dependent ROS Generation”, Int J Nanomedicine., 2020, 15, 3695–3716. |
| 7 | Cell:C2C12, myocytes | – | Z. Jing, T. Iba, H. Naito, P. Xu, J.I. Morishige, N. Nagata, H. Okubo and H.Ando ,”L-carnitine prevents lenvatinib-induced muscle toxicity without impairment of the anti-angiogenic efficacy”, Front Pharmacol., 2023, doi: 10.3389/fphar.2023.1182788. |
| 8 | Cell:C2C12, 3T3L1 | Plate reader | M. Kurano, K. Tsukamoto, T. Shimizu, H. Kassai, K. Nakao, A. Aiba, M. Hara and Yatomi , “Protection Against Insulin Resistance by Apolipoprotein M/Sphingosine 1-Phosphate “, Diabetes, 2020, DOI: 10.2337/db19-0811. |
| 9 | Cell:Colon 26 | Microscope | B. Uranbileg, M. Kurano, K. Kano, E. Sakai, J. Arita, K. Hasegawa, T. Nishikawa, S. Ishihara, H. Yamashita, Y. Seto, H. Ikeda, J. Aoki and Y. Yatomi,”Sphingosine 1‐phosphate lyase facilitates cancer progression through converting sphingolipids to glycerophospholipids”, Clin Transl Med., 2022, doi: 10.1002/ctm2.1056. |
| 10 | Tissue: Frozen heart slides |
Microscope | W. Yu, Y. Hu, Z. Liu, K. Guo, D. Ma, M. Peng, Y. Wang, J. Zhang, X. Zhang, P. Wang, J. Zhang, P. Liu and J. Lu,”Sorting nexin 3 exacerbates doxorubicin-induced cardiomyopathy via regulation of TFRC-dependent ferroptosis”, Acta Pharmaceutica Sinica B., 2023, doi: https://doi.org/10.1016/j.apsb.2023.08.016. |
| 11 | Cell:HCE | Microscope | T. Yamashita, K. Asada, M. Ueno, N. Hiramoto, T. Fujita, M. Toda, C. Sotozono, S. Kinoshita and J. Hamuro,”Cellular interplay through extracellular vesicle miR-184 alleviates corneal endothelium degeneration”, Ophthalmol Sci., 2022, doi: 10.1016/j.xops.2022.100212. |
| 12 | Cell:HCE | Microscope | M. Ueno, K Yoshii, T. Yamashita, K. Sonomura, K. Asada, E. Ito, T. Fujita, C. Sotozono, S. Kinoshita and J. Hamuro,”The Interplay Between Metabolites and MicroRNAs in Aqueous Humor to Coordinate Corneal Endothelium Integrity”, Ophthalmol Sci., 2023, doi: 10.1016/j.xops.2023.100299. |
| 13 | Cell:HCE-T | – | W. Otsu, T. Yako, E. Sugisawa, S. Nakamura, H. Tsusaki, N. Umigai, M. Shimazawa and H. Hara,”Crocetin protects against mitochondrial damage induced by UV-A irradiation in corneal epithelial cell line HCE-T cells”, J Pharmacol Sci., 2022, doi: 10.1016/j.jphs.2022.10.005. |
| 14 | Cell:HCE-T | Microscope | K. Ishida, T. Yako, M. Tanaka, W. Otsu, S. Nakamura, M. Shimazawa, H. Tsusaki and H. Hara,”Free-radical scavenger NSP-116 protects the corneal epithelium against UV-A and blue led light exposure”, Biol Pharm Bull., 2021, doi: 10.1248/bpb.b21-00017. |
| 15 | Cell:HepG | Microscope/Spectrophotometer | M. Ikura, K. Furuya, T. Matsuda and T. Ikura,”Impact of Nuclear De Novo NAD+ Synthesis via Histone Dynamics on DNA Repair during Cellular Senescence To Prevent Tumorigenesis”, Mol Cell Biol., 2022, doi: 10.1128/mcb.00379-22. |
| 16 | Cell:hiPSCs, Neurons | Microscope | T. Hara, M. Toyoshima, Y. Hisano, S. Balan, Y. Iwayama, H. Aono,Y. Futamura, H. Osada, Y. Owada and T. Yoshikawa,”Glyoxalase I disruption and external carbonyl stress impair mitochondrial function in human induced pluripotent stem cells and derived neurons”, Translational Psychiatry., 2021, doi: 10.1038/s41398-021-01392-w. |
| 17 | Cell:HSCs | Microscope | Y. Su, S. Lu, C. Hou, K. Ren, M. Wang, X. Liu, S. Zhao and X. Liu ,”Mitigation of liver fibrosis via hepatic stellate cells mitochondrial apoptosis induced by metformin”, International Immunopharmacology., 2022, doi: 10.1016/j.intimp.2022.108683. |
| 18 | Cell:HUVECs | Microscope | D. Ueno, K. Ikeda, E. Yamazaki, A. Katayama, R. Urata and S. Matoba ,”Spermidine improves angiogenic capacity of senescent endothelial cells, and enhances ischemia-induced neovascularization in aged mice”, Sci Rep., 2023, doi: 10.1038/s41598-023-35447-3. |
| 19 | Cell:KYSE30 | Microscope | Q. Luo, X. Wu, P. Zhao, Y. Nan, W. Chang, X. Zhu, D. Su and Z. Liu,”OTUD1 activates caspase‐independent and caspase‐dependent apoptosis by promoting AIF nuclear translocation and MCL1 degradation”, Adv Sci (Weinh)., 2021, doi: 10.1002/advs.202002874. |
| 20 | Cell: Macrophage | Microscope | G. Yang, M. Fan, J. Zhu, C. Ling, L. Wu, X. Zhang, M. Zhang, J. Li, Q. Yao, Z. Gu and X. Cai, “A multifunctional anti-inflammatory drug that can specifically target activated macrophages massively deplete intracellular H2O2 and produce large amounts CO for a highly efficient treatment of osreoarthritis” , Biomaterials, 2020, doi:10.1016/j.biomaterials.2020.120155. |
| 21 | Cell:MDA-MB-415, MCF-7 | Microscope | S.Y. Park, K.J. Jeong, A. Poire, D. Zhang, Y.H. Tsang, A.S. Blucher and G.B. Mills ,”Irreversible HER2 inhibitors overcome resistance to the RSL3 ferroptosis inducer in non-HER2 amplified luminal breast cancer”, Cell Death & Disease., 2023, doi: 10.1038/s41419-023-06042-1. |
| 22 | Cell:MIN6 | Plate reader/Microscope | N. Mizusawa, N. Harada, T. Iwata, I. Ohigashi, M. Itakura and K. Yoshimoto,”Identification of protease serine S1 family member 53 as a mitochondrial protein in murine islet beta cells”, Islets., 2022, doi: 10.1080/19382014.2021.1982325. |
| 23 | Cell:MSCs | Flow Cytometer | S.Y. Jo, H.J. Cho and T.M. Kim,”Fenoldopam mesylate enhances the survival of mesenchymal stem cells under oxidative stress and increases the therapeutic function in acute kidney injury”, Cell Transplant., 2023, doi: 10.1177/09636897221147920. |
| 24 | Cell:Neuro-2A | Microscope、Plate reader | Y. Wang, Y. Shinoda, A. Cheng, I. Kawahata and K. Fukunaga,”Epidermal fatty acid-binding protein 5 (FABP5) Involvement in alpha-synuclein-induced mitochondrial injury under oxidative stress”, Biomedicines., 2021, doi: 10.3390/biomedicines9020110. |
| 25 | Cell:Neuron | Microscope | I. Kawahata, L. Luc Bousset, R. Melki and K. Fukunaga , “Fatty Acid-Binding Protein 3 is Critical for α-Synuclein Uptake and MPP+-Induced Mitochondrial Dysfunction in Cultured Dopaminergic Neurons “, Int J Mol Sci., 2019, 20, 5358. |
| 26 | Cell:Neuron | Microscope | A. Fukuda, S. Nakashima,Y. Oda, K. Nishimura, H. Kawashima, H. Kimura, T. Ohgita, E. Kawashita, K. Ishihara, A. Hanaki, M. Okazaki, E. Matsuda, Y. Tanaka, S. Nakamura, T. Matsumoto, S. Akiba, H. Saito, H. Matsuda and K. Takata,”Plantainoside B in Bacopa monniera Binds to Aβ Aggregates Attenuating Neuronal Damage and Memory Deficits Induced by Aβ”, Biol Pharm Bull., 2023, doi: 10.1248/bpb.b22-00797. |
| 27 | Cell:PAECs | Plate reader | T. Sakai, H. Takagaki, N. Yamagiwa, M. Ui, S. Hatta and J. Imai,”Effects of the cytoplasm and mitochondrial specific hydroxyl radical scavengers TA293 and mitoTA293 in bleomycin-induced pulmonary fibrosis model mice”, Antioxidants (Basel)., 2021, doi: 10.3390/antiox10091398. |
| 28 | Cell:PANC-1 | Plate reader | W.A. Naime, A. Kimishima, A. Setiawan, J.R. Fahim, M.A. Fouad, M.S. Kamel and M. Arai,”Mitochondrial Targeting in an Anti-Austerity Approach Involving Bioactive Metabolites Isolated from the Marine-Derived Fungus Aspergillus sp.”, Marine drugs., 2020, doi: 10.3390/md18110555. |
| 29 | Cell:PANC-1, MIAPaca-2 | Microscope | T. Taniai, Y. Shirai,Y. Shimada, R. Hamura, M. Yanagaki, N. Takada, T. Horiuchi, K. Haruki, K. Furukawa, T. Uwagawa, K. Tsuboi, Y. Okamoto, S. Shimada, S. Tanaka, T. Ohashi and T. Ikegami,”Inhibition of acid ceramidase elicits mitochondrial dysfunction and oxidative stress in pancreatic cancer cells”, Cancer Sci., 2021, doi: 10.1111/cas.15123. |
| 30 | Cell:PC | Flow Cytometer | R. Hamura, Y. Shirai,Y. Shimada, N. Saito, T. Taniai, T. Horiuchi, N. Takada, Y. Kanegae, T. Ikegami, T. Ohashi and K. Yanaga ,”Suppression of lysosomal acid alpha‐glucosidase impacts the modulation of transcription factor EB translocation in pancreatic cancer”, Cancer Sci., 2021, doi: 10.1111/cas.14921. |
| 31 | Cell:Porcine oocytes | Microscope | W. Hu, Y. Zhang, D. Wang, T. Yang, J. Qi, Y. Zhang, H. Jiang, J Zhang, B. Sun and S. Liang,”Iron Overload-Induced Ferroptosis Impairs Porcine Oocyte Maturation and Subsequent Embryonic Developmental Competence in vitro”, Front Cell Dev Biol., 2021, doi: 10.3389/fcell.2021.673291. |
| 32 | Cell:Porcine oocytes | Microscope | Y. Xiao, B. Yuan, W. Hu, J. Qi, H. Jiang, B. Sun, J. Zhang and S. Liang,”Tributyltin Oxide Exposure During in vitro Maturation Disrupts Oocyte Maturation and Subsequent Embryonic Developmental Competence in Pigs”, Front Cell Dev Biol., 2021, doi: 10.3389/fcell.2021.683448. |
| 33 | Cell:RGC-5 | Plate reader | Y. Aoyama, S. Inagaki, K. Aoshima, Y. Iwata, S. Nakamura, H. Hara and M. Shimazawa,”Involvement of endoplasmic reticulum stress in rotenone-induced leber hereditary optic neuropathy model and the discovery of new therapeutic agents”, J Pharmacol Sci . .,2021, doi: 10.1016/j.jphs.2021.07.003. |
| 34 | Cell:SAS,HSC-2 | Plate reader | K. Yamana, J. Inoue, R. Yoshida, J. Sakata, H. Nakashima, H. Arita, S. Kawaguchi, S. Gohara, Y. Nagao, H. Takeshita, M. Maeshiro, R. Liu, Y. Matsuoka, M. Hirayama, K. Kawahara, M. Nagata, A. Hirosue, R. Toya, R. Murakami, Y. Kuwahara, M. Fukumoto and H. Nakayama,”Extracellular vesicles derived from radioresistant oral squamous cell carcinoma cells contribute to the acquisition of radioresistance via the miR‐503‐3p‐BAK axis”, J Extracell Vesicles., 2021, doi: 10.1002/jev2.12169. |
| 35 | Cell:SBC-3 | Flow Cytometer | N. Takahashi, T. Iguchi, M. Kuroda, M. Mishima and Y. Mimaki,”Novel Oleanane-Type Triterpene Glycosides from the Saponaria officinalis L. Seeds and Apoptosis-Inducing Activity via Mitochondria”, Int J Mol Sci., 2022, doi: 10.3390/ijms23042047. |
| 36 | Cell:SH-SY5Y | Microscope | Q. Guo, I. Kawahata, A. Cheng, H. Wang, W. Jia, H. Yoshino and K. Fukunaga,”Fatty acid-binding proteins 3 and 5 are involved in the initiation of mitochondrial damage in ischemic neurons”, Redox Biology., 2023, doi: 10.1016/j.redox.2022.102547. |
| 37 | Cell:SiHa | Microscope | F.F. Gao, J.H. Quan, M.A. Lee, W. Ye, J.M. Yuk, G.H. Cha, I.W. Choi and Y.H. Lee,”Trichomonas vaginalis induces apoptosis via ROS and ER stress response through ER–mitochondria crosstalk in SiHa cells”, Parasites &vectors., 2021, doi: 10.1186/s13071-021-05098-2. |
| 38 | Cell:SU-DHL-2 | Flow Cytometer | Q. Zhao, D. Jiang, X. Sun, Q. Mo, S. Chen, W. Chen, R. Gui and X. Ma,”Biomimetic nanotherapy: core–shell structured nanocomplexes based on the neutrophil membrane for targeted therapy of lymphoma”, J Nanobiotechnology., 2021, doi: 10.1186/s12951-021-00922-4. |
| 39 | Cell:THP-1 | Microscope | W. Zheng, Z. Zhou, Y. Rui, R. Ye, F. Xia, F. Guo, X. Liu, J. Su, M. Lou, and X.F. Yu,”TRAF3 activates STING-mediated suppression of EV-A71 and target of viral evasion”, Signal Transduct Target Ther., 2023, doi: 10.1038/s41392-022-01287-2. |
| 40 | Cell:TSM15 | In Cell Analyzer | M. Honda, F. Shimizu, R. Sato, Y. Mizukami, K. Watanabe, Y. Takeshita, T. Maeda, M. Koga and T. Kanda,”Jo-1 Antibodies From Myositis Induce Complement-Dependent Cytotoxicity and TREM-1 Upregulation in Muscle Endothelial Cells”, Neurol Neuroimmunol Neuroinflamm., 2023, doi: 10.1212/NXI.0000000000200116. |
| 41 | Cell:tumor | Flow Cytometer | H. Wang, X. Rong, G. Zhao, Y. Zhou, Y. Xiao, D. Ma, X. Jin, Y. Wu, Y. Yan, H. Yang, Y. Zhou, M. Qian, C. Niu, X. Hu, D.Q. Li, Q. Liu, Y. Wen, Y.Z. Jiang, C. Zhao and Z.M. Shao ,”The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer”, Cell Metab., 2022, doi: 10.1016/j.cmet.2022.02.010. |
| 42 | Cell:TY10 | In Cell Analyzer | F. Shimizu, R. Ogawa, Y. Mizukami, K. Watanabe, K. Hara, C. Kadono, T. Takahashi, T. Misu, Y. Takeshita, Y. Sano, M. Fujisawa, T. Maeda, I. Nakashima, K. Fujihara and T. Kanda,”GRP78 antibodies are associated with blood-brain barrier breakdown in anti–myelin oligodendrocyte glycoprotein antibody–associated disorder”, Neurol Neuroimmunol Neuroinflamm., 2022, doi: 10.1212/NXI.0000000000001038. |
| 43 | Cell:U2OS, HeLa | Microscope | T. Namba, “BAP31 regulates mitochondrial function via interaction with Tom40 within ER-mitochondria contact sites “, Sci Adv., 2019, 5, (6), 1386. |
常见问题Q&A
| Q1: 本试剂盒可以检测多少次? |
| A1:大概的使用次数请参考下表: |
| 检测装置 | 容器 | 使用次数 | 液量 |
| 流式细胞仪 | – | 100次 | 0.5 ml/次 |
| 荧光显微镜 荧光酶标仪 |
35 mm dish | 25块板 | 2 ml/孔 |
| 8孔Chamber Slide | 30块板 | 200 μl/孔 | |
| 96孔板 | 5块板 | 100 μl/孔 |
| Q2:在JC-1染色后,可以使用PBS代替HBSS洗涤吗? |
| A2:我们建议使用HBSS来减少对细胞的损伤。如果您手边没有HBSS的话,建议使用培养基洗净。 |
| Q3:可以使用含血清的培养基吗? |
| A3:在清洗细胞和Working Solution中可以使用含血清的培养基。在观察荧光时建议使用Imaging Buffer。如果一定要使用含血清的培养基的话,建议不要加酚红。 |
| Q4:染色后细胞固定或者固定后进行染色可以实现吗? |
| A4:细胞固定操作会使得线粒体去极化,所以染色前后均不能进行细胞固定。 |
|
Q5:处理后的样品与对照组相比较,红和绿两种荧光值都增加(或减少)了,结果该如何解释? |
| A5:请先比较实验组和对照组的荧光比值,两者相比,荧光比越低,线粒体膜电位越低。
用荧光之比进行结果分析的理由。 JC-1由于膜电位依存性地在细胞中积蓄,根据细胞的状态,每个细胞的JC-1的浓度有可能不同。 由于对照组和实验组处理样品的细胞状态不同,JC-1的累积浓度不同。) 另外,在线粒体膜电位较高的状态下,JC-1会聚集在一起,使荧光从绿色转移到红色。 该聚集体的量取决于膜电位的程度,因此可以用红/绿之比来比较样品之间的线粒体膜电位。 <参考文献> 1) Cossarizza, A. et al., Biochem Biophys Res Commun., 1993, 197(1), 40. 2) Perelman, A. et al., Cell Death and Disease, 2012, 3, e430 3) Smiley, S. T. et al., Proc. Nail. Acad. Sci., 1991, 88, 3671. |
关联产品