上海金畔生物科技有限公司提供天然产物萜类及其苷类Terpenoids and Glycosides。
Simvastatin (Synonyms: 辛伐他汀; MK 733) 纯度: 99.45%
Simvastatin (MK 733) 是一种竞争性的 HMG-CoA reductase 抑制剂,Ki 值为 0.2 nM。
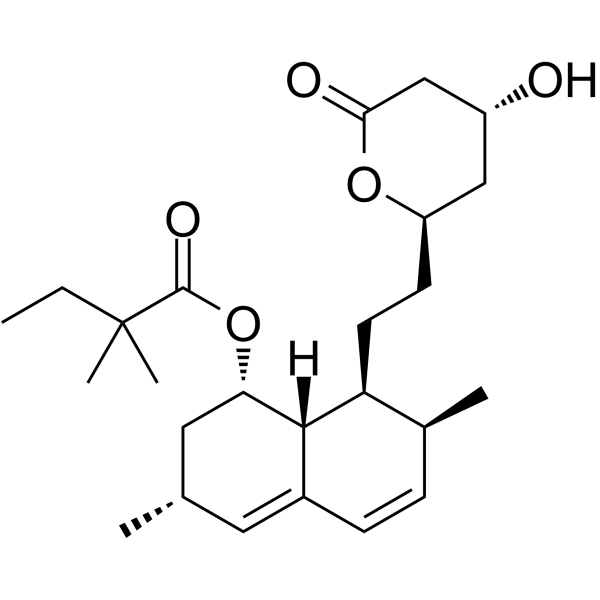
Simvastatin Chemical Structure
CAS No. : 79902-63-9
| 规格 | 价格 | 是否有货 | 数量 |
|---|---|---|---|
| Free Sample (0.1-0.5 mg) | Apply now | ||
| 10 mg | ¥450 | In-stock | |
| 50 mg | ¥800 | In-stock | |
| 100 mg | ¥1400 | In-stock | |
| 200 mg | ¥2400 | In-stock | |
| 500 mg | ¥4800 | In-stock | |
| 1 g | 询价 | ||
| 5 g | 询价 |
* Please select Quantity before adding items.
Simvastatin 相关产品
•相关化合物库:
- Drug Repurposing Compound Library Plus
- FDA-Approved Drug Library Plus
- FDA-Approved Drug Library Mini
- Bioactive Compound Library Plus
| 生物活性 |
Simvastatin (MK 733) is a competitive inhibitor of HMG-CoA reductase with a Ki of 0.2 nM. |
IC50 & Target |
Ki: 0.2 nM (HMG-CoA reductase)[1] |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 体外研究 (In Vitro) |
Simvastatin needs to be activated by NaOH in EtOH treatment before use for cell assay (Activation of Simvastatin: 2 mg of Simvastatin in 50 μL of ethanol and 75 μL of 0.1 N NaOH, incubated at 50°C for 2 hours. The pH is adjusted to 7.2 with HCl). Simvastatin suppresses cholesterol synthesis in mouse L-M cell, rat H4II E cell, and human Hep G2 cell with IC50s of 19.3 nM, 13.3 nM and 15.6 nM, respectively[1]. Simvastatin causes a dose-dependent increase in serine 473 phosphorylation of Akt within 30 minutes, with maximal phosphorylation occurring at 1.0 µM. Simvastatin (1.0 μM) enhances phosphorylation of the endogenous Akt substrate endothelial nitric oxide synthase (eNOS), inhibits serum-free media undergo apoptosis and accelerates vascular structure formation[2]. Simvastatin shows anti-inflammatory effects, reduces anti-CD3/anti-CD28 antibody-stimulated proliferation of PB-derived mononuclear cells and synovial fluid cells from rheumatoid arthritis blood, as well as IFN-γ release at 10 μM. Simvastatin (10 μM) also blocks cell-mediated macrophage TNF-γ release induced via cognate interactions by appr 30%[3]. Simvastatin (5 μM) significantly reduces the expression of ABCA1 in astrocytes and neuroblastoma cells, the expression of apolipoprotein E in astrocytes, and increases cyclin-dependent kinase 5 and glycogen synthase kinase 3β expression in SK-N-SH cells[7].Simvastatin has the ability to inhibit exosome release[10]. Shanghai Jinpan Biotech Co Ltd has not independently confirmed the accuracy of these methods. They are for reference only. |
||||||||||||||||
| 体内研究 (In Vivo) |
Simvastatin suppresses the conversion of radiolabeled acetate to cholesterol with an IC50 of 0.2 mg/kg via p.o. administration[1]. Simvastatin (4 mg/day, p.o. for 13 weeks) returns the cholesterol-induced increases in total cholesterol, LDL-cholesterol and HDL-cholesterol to normal level in rabbits fed an atherogenci cholesterol-rich diet[4]. Simvastatin (6 mg/kg) increases LDL receptor-dependent binding and the number of hepatic LDL receptors in rabbits fed a diet containing 0.25% cholesterol[5]. Simvastatin affects inflammation independent of its effect on plasma cholesterol level. Simvastatin (20 mg/kg/day) causes a 1.3-fold less macrophage content in lesions, and 2-fold less vascular cell adhesion molecule-1, interleukin-1beta, and tissue factor expression, companied by a 2.1-fold increases in lesional smooth muscle cell and collagen content in cynomolgus monkeys fed an atherogenic diet[6]. Shanghai Jinpan Biotech Co Ltd has not independently confirmed the accuracy of these methods. They are for reference only. |
||||||||||||||||
| Clinical Trial |
|
||||||||||||||||
| 分子量 |
418.57 |
||||||||||||||||
| Formula |
C25H38O5 |
||||||||||||||||
| CAS 号 |
79902-63-9 |
||||||||||||||||
| 中文名称 |
辛伐他汀;辛伐他丁 |
||||||||||||||||
| 运输条件 |
Room temperature in continental US; may vary elsewhere. |
||||||||||||||||
| 储存方式 |
|
||||||||||||||||
| 溶解性数据 |
In Vitro:
Ethanol : 100 mg/mL (238.91 mM; Need ultrasonic) DMSO : ≥ 50 mg/mL (119.45 mM) * “≥” means soluble, but saturation unknown. 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂: ——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用; 以下溶剂前显示的百
|
||||||||||||||||
| 参考文献 |
|
| Cell Assay [7] |
Before use, Simvastatin needs to be converted to open acid form by reconstitution of 50mg in 1mL ethanol, addition of 0.813 mL 1N NaOH, and pH adjustment to 7.2 with 1N HCl[9]. Primary human astrocytes from four different donors are prepared from tissue obtained from legally aborted fetuses. Primary human astrocytes and SK-N-SH neuroblastoma cell line (ATCC) are plated on 6-well plates and grown in DMEM supplemented with 5% or 8% fetal calf serum, respectively, at 37°C, 5% CO2 until 80% confluent. For measurement of gene expression levels at baseline, cells are just washed and RNA is prepared and assayed as outlined below. Baseline gene expression levels in astrocytes are measured in primary human astrocytes obtained from two donors. For experimental purposes, cells are incubated under serum-free conditions. Primary human astrocytes are obtained from four donors. Based on preliminary time-dependent studies, 48-h incubation is used for all of experiments. Based on the dose-response studies, a majority of our experiments are conducted using the following concentrations of active compounds: Simvastatin at 5 μM, CS-514 at 10 μM, mevalonate at 50 μM, and GGPP and FPP at 10 μM. Following incubation, cells are extensively washed to remove dead cells and cell debris and prepared for further analyses[7]. Shanghai Jinpan Biotech Co Ltd has not independently confirmed the accuracy of these methods. They are for reference only. |
|---|---|
| Animal Administration [1] |
Monkeys[1] Shanghai Jinpan Biotech Co Ltd has not independently confirmed the accuracy of these methods. They are for reference only. |
| 参考文献 |
|
